Table of Contents
Important Topic of Chemistry: Ozonolysis
Ozonolysis is an organic reaction in which ozone cleaves the unsaturated bonds of alkenes, alkynes, or azo compounds. By replacing several carbon-carbon bonds with a carbonyl group, alkenes and alkynes generate organic compounds, whereas azo compounds form nitrosamines. The reaction’s outcome is determined by the type of multiple bonds being oxidized as well as the work-up conditions. Inorganic chemistry, ozonolysis is a reaction used to determine the position of a carbon-carbon double bond in unsaturated compounds. It entails reacting the compound with ozone to form an ozonide, and the ozonide yields a mixture containing aldehydes, ketones, or carboxylic acids after hydrogenation or acid treatment. The position of the double bond in the original unsaturated compound can be determined by determining the structure of the aldehydes and ketones produced. Ozonolysis has been widely used to determine the structure of natural products, particularly terpenes. It has also been used to investigate the structure of aromatic compounds as well as to synthesize rare aldehydes and ketones. On ozonolysis, oleic acid produces n-nonanal (pelargonic aldehyde) and azelaic semialdehyde. Ozonolysis is the cleavage of alkene double bonds by ozone reaction. Different products can be isolated depending on the work-up: reductive workup yields alcohols or carbonyl compounds, whereas oxidative workup yields carboxylic acids or ketones.
Overview
Ozonolysis is an organic redox reaction in which ozone cleaves unsaturated carbon-carbon bonds (either double or triple bonds) in alkenes, alkynes, or azo compounds. Azo compounds are those that have the functional group diazenyl (R-N=N-R’). This reaction has a variety of applications, including the production of alcohols, aldehydes, ketones, carboxylic acids, and so on. Ozonolysis is a type of organic chemistry reaction used to determine the position of a carbon-carbon double bond in unsaturated compounds. Ozonolysis is the reaction of a compound with ozone that results in the formation of an ozonide, and the ozonide yields a mixture containing aldehydes, ketones, or carboxylic acids when hydrogenated or treated with acid. The mechanism of ozonolysis is an oxidative cleavage reaction. The ozone not only breaks the carbon pi bond, but also the carbon-carbon sigma bond. This mechanism involves the formation of an ozonide by the attack of ozone on the given reactant. Zinc dust is used to remove the oxygen content in this intermediate stage, even though it forms zinc oxide with the oxygen. The final product may differ depending on the type of reactant and the workup. Alcohols, aldehydes, ketones, and carboxylic acids can be produced by oxidizing alkenes with ozone. Ozonolysis converts alkynes into acid anhydrides or diketones.
If there is water in the reaction, the acid anhydride hydrolyzes to produce two carboxylic acids. Ozone cracking is another name for elastomer ozonolysis. The presence of trace amounts of ozone gas in the atmosphere weakens the double bonds in elastomers. Ozonolysis produces nitrosamines from azo compounds.
Ozonolysis reaction
Ozone is a highly reactive oxygen allotrope. When ozone reacts with alkenes and alkynes, the alkene or alkyne is oxidatively cleaved. The carbon-carbon triple bonds are replaced by carbon-oxygen double bonds, resulting in the required carbonyl product, as shown below.
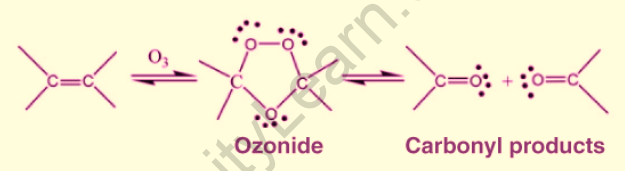
Ozonolysis Mechanism
The mechanism of ozonolysis is based on an oxidative cleavage reaction. Ozone not only breaks the carbon pi bond, but it also breaks the carbon-carbon sigma bond. It entails the formation of an ozonide by the attack of ozone on the given reactant. Zinc dust is used to remove the oxygen in this intermediate stage (since it forms zinc oxide with oxygen). The final product varies depending on the reactant and the workup. The diagram below depicts the general mechanism of the ozonolysis reaction for alkenes and alkynes.
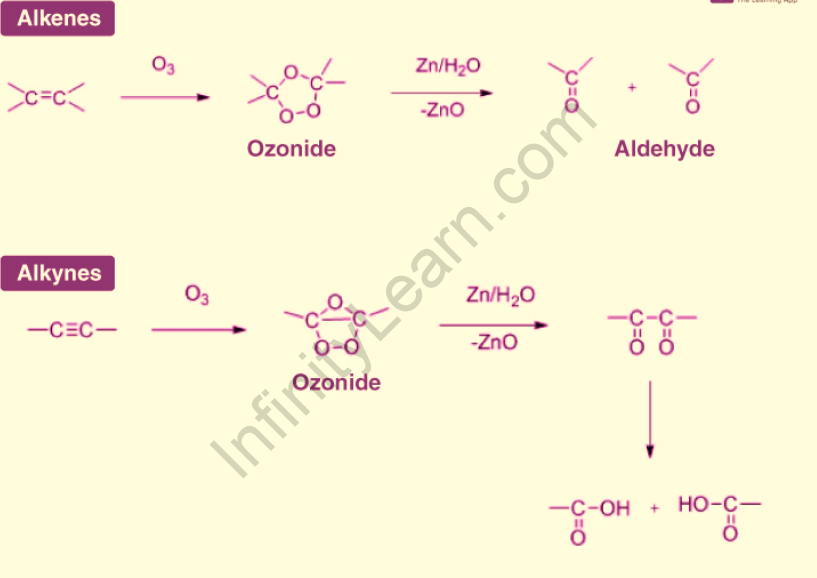
Ozonolysis of alkenes
Ozonolysis can convert alkenes into alcohols, aldehydes, ketones, or carboxylic acids. A solution of alkene in methanol is used in the general procedure. At around 780 degrees Celsius, ozone is bubbled through this solution. The alkene is consumed when the solution turns blue (the blue colour comes from the unreacted ozone). Potassium iodide solution is another indicator of the reaction’s endpoint. A stream of ozone-enriched oxygen is bubbled through a mixture of the reactants to create this indicator. The gas that bubbles out is routed through a potassium iodide solution. When the reaction is finished and there is no more alkene to react with the ozone, the gas proceeds to oxidize potassium iodide to iodine. The distinct violet colour of iodine indicates the reaction of potassium iodide with ozone to produce iodine.
After adding the ozone to the reaction mixture, a reagent must be added to convert the ozonide to the required carbonyl derivative.
Two techniques can be used for this conversion:
- Workout for Reduction
- Workout for Oxidative Stress
Carbon atoms are still held together by a single bond in this structure. It will then rearrange itself to form ozonide, a relatively stable intermediate. The bond between two carbon atoms is broken in the ozonide intermediate. The carbon atoms in the ozonide intermediate are linked directly by oxygen atoms. In the presence of dimethyl sulphide and zinc dust, ozonide will then be reduced. The reduction reaction produces two products, both of which contain a double bond between carbon and oxygen atoms, referring to the ozonolysis reaction. The lysis of a double bond in the presence of an ozone molecule is known as ozonolysis. After ozonolysis of alkenes, the products formed would be ketones, alcohols, aldehydes, or carboxylic acids.
Ozonolysis of alkynes
The final product of ozonolysis on alkynes is an acid anhydride or diketone. This reaction’s fragmentation is incomplete (alkenes undergo complete fragmentation). A simple aqueous workup is used, so no reducing agents are required. If the reaction occurs in the presence of water, the acid anhydride hydrolyzes to yield two carboxylic acids. Ozonolysis can also be used to determine the triple bond position in an unknown alkyne. Here are a few examples of alkyne ozonolysis:
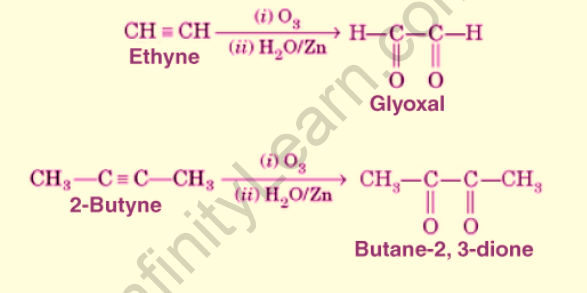
Alkynes are made up of two pi bonds. In the presence of an ozone molecule, the triple bond of alkynes undergoes oxidative cleavage. Alkynes are oxidised, resulting in the formation of end products such as diketones and acid anhydrides. Essentially, in the presence of water, the acid anhydride hydrolyzes to produce two carboxylic acids. In the presence of ozone molecules, the alkyne is typically subjected to ozonolysis. The negatively charged oxygen atom will attack one end of the carbon-carbon triple bond. The positively charged oxygen atom will attack the carbon-carbon triple bond’s other end. An intermediate with a carbon-carbon double bond is formed, which is unstable and undergoes rearrangement. A stable ozonide is formed when a double bond between two carbon atoms is broken. The triple bond is reduced to a single bond in ozonide. The single bond between two carbon atoms then breaks, resulting in a diketone compound. The diketone compound will then undergo hydrolysis, yielding two carboxylic acids.
An alternative to using ozone for oxidative workup is to use the reagent KMnO4, particularly in the presence of hot acid; the result will be the same. This is the final category of important alkene reactions we’ll cover in this series for the time being; in the next post, we’ll wrap up the alkene reactions with a summary post.
Also read: Important Topic of Chemistry: Oxidation
FAQs
What are some of the applications of Ozonolysis?
Ozonolysis is used in organic chemistry to determine the location of double or triple bonds in alkenes and alkynes, respectively. Ozonolysis is a technique used to determine the structure of long alkenes and alkynes. Bleaching employs ozonolysis. Ozonolysis is used in wastewater treatment. Ozonolysis is used to produce alcohols, carboxylic acids, aldehydes, and ketones.
What is the ozonolysis mechanism?
The ozonolysis mechanism divides reactions into three steps. 1: Ozone molecule attack - The ozone molecule is made up of three oxygen atoms, one of which has a partial negative charge and the other has a partial positive charge. 2: Ozonide intermediate formation - It rearranges itself, resulting in the formation of a stable ozonide intermediate. 3: Carbonyl compound formation - In the presence of zinc dust, two carbonyl compounds are formed.
What exactly is ozonolysis?
Ozonolysis is a type of organic chemistry reaction used to determine the position of a carbon-carbon double bond in unsaturated compounds. Ozonolysis is the reaction of a compound with ozone that results in the formation of an ozonide, and the ozonide yields a mixture containing aldehydes, ketones, or carboxylic acids when hydrogenated or treated with acid.





