Table of Contents
Introduction:
Solutions are a part of our daily lives since they are present in every ordinary object you use in your daily life, such as soda, deodorant that you put on, sugar, salt, and so on. A solution is essentially a sort of combination in which two or more chemicals combine to produce a single solution.
A solution is a homogenous combination of two or more components with particle sizes less than one nanometer.
Sugar and salt solutions in water, as well as soda water, are popular examples of solutions. All of the components appear as a single phase in a solution. There is particle homogeneity, which means that the particles are uniformly dispersed. This is why a full bottle of soft drink tastes the same.
A homogeneous solution is a homogeneous mixture of two or more components. During the formation of a solution, any state of matter (solid, liquid, or gas) can function as both a solvent and a solute. As a result, depending on the physical states of the solute and solvent, there are nine distinct types of solutions.
Components of Solution
A solution is composed of two components: a solvent and a solute.
The component that dissolves the other component is known as the solvent.
The term “solute” refers to the component(s) dissolved in the solvent (s).
In general, the solvent predominates over the solute. The solute amount is less than the solvent amount. Solutes and solvents may be found in all states of matter, including solids, liquids, and gases.
A liquid solution is made up of a solid, liquid, or gas that has been dissolved in a liquid solvent. Solid and gaseous solutions are represented by alloys and air, respectively.
Examples: The examples below show the solvent and solute in several solutions.
- Sugar syrup is a solution made by dissolving sugar in water with heat. Water is the solvent in this case, while sugar is the solute.
- Air is a gaseous mixture that is homogenous. Both the solvent and the solute are gases in this case.
- Iodine tincture is an iodine-in-alcohol solution. The solute is iodine, while the solvent is alcohol.
Solution Characteristics
The following are the many qualities of solutions:
- Solutes are indistinguishable from the mixture and do not settle. A solution is dependable.
- Filtration cannot separate the components of a mixture.
- It is a completely homogenous combination.
- Its particles are too small, with a diameter of less than 1 nm.
- The particles cannot be seen with the naked eye.
- Particles do not disperse a light beam travelling through them, hence the path of the light is not apparent.
Different Types of Solutions
There are several types of solutions that may be categorized based on factors such as the difference in solute and solvent, the number of criteria, and so on, as shown below:
Various types of solutions based on water as a solvent
Solutions are classed into two categories based on whether or not they include water.
- Aqueous Solution
The solution in which any homogenous substance entirely dissolves in water, with water acting as a solvent. Sugar/salt in water and carbon dioxide in water are two examples of this type of solution.
- Non-Aqueous Solution
These solutions are essentially the inverse of Aqueous solutions in that the solvent present is not water; it may be anything else such as petrol, benzene, ether, and so on. This type of solution includes phenolphthalein in benzene, sulfur in carbon disulfide, and others.
Various sorts of solutions based on the amount of solute supplied
On the basis of the quantity of solute contained in the solution, solutions are divided into three categories.
- Saturated Solutions
At a specific temperature, a solution is said to be saturated if it no longer has the ability to dissolve any more solute in the solvent.
- Unsaturated Solutions
If the solution can still dissolve additional solute in the solvent, it is considered to be unsaturated.
- Supersaturated solutions
Supersaturated solutions are those in which the solute is present in excess and is forcedly dissolved in the solvent by raising the temperature. With the aid of the crystallization process, these extra solute particles are eventually found in the form of crystals.
Various sorts of solutions based on the amount of solvent added
On the basis of the amount of solvent contained in the solution, solutions are categorized into two categories.
- Concentrated Solutions
To produce concentrated solutions, a large amount of solute is added to the provided solvent.
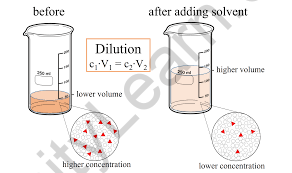
- Diluted Solutions
Dilute solutions are solutions that include a little quantity of solute in a big volume of solvent.
Different sorts of solutions based on the quantity of solute concentration in two solutions
Based on the concentration of the solvent in two solvents (in a beaker and a cell in it), solutions may be categorized into three categories.
- Hypertonic solutions
Hypertonic solutions are ones in which the concentration of the solute in a beaker is greater than that in the cell, causing water to exit the cell and cause the cell to plasmolyze/ shrink.
- Hypotonic Solutions
Hypotonic solutions are ones in which the concentration of solute in a beaker is lower than that in the cell, allowing water to migrate into the cell and cause it to inflate and burst.
- Isotonic Solutions
Because the solute content in both the beaker and the cell is the same, water will travel in both directions around the cell.
Solutions are classified based on their capacity to conduct electric current; those containing molecules are known as non-conductors, while those containing ions are known as conductors.
Electrolytes are compounds that dissolve in water and form ions, whereas non-electrolytes are substances that dissolve in water but do not form ions. These ion-forming compounds, known as electrolytes, transmit electric current in liquids and are further categorized into Strong electrolytes and Weak electrolytes.
- Strong Electrolyte
Strong electrolytes are only accessible in the form of ions, which causes the light bulb on the conductivity device to shine vividly (which is used to check the electric current in the solution). An excellent example of a Strong electrolyte is NaCl.
- Weak Electrolyte
Weak electrolytes are solutions that contain only a few ions and cause the light bulb on the conductivity instrument to glow weakly. Weak acids and bases are excellent examples of weak electrolytes.
FAQ’s
Describe the circumstances that must be met in order for an ideal solution to be developed.
If an ideal solution is to be constructed, the key requirement that must be met is a solution that obeys Raoult's law. Furthermore, the solution must be homogeneous and free of any volumetric or thermal effects.
A solution of chlorobenzene and bromobenzene is approximately optimal, but a solution of chloroform and acetone is not. Why?
In the case of a combination of chlorobenzene and bromobenzene, the molecules interact equally, resulting in a perfect solution. They are not present in the chloroform-acetone combination.









