In chemistry, pH, traditionally denoting “potential of hydrogen” (or “power of hydrogen”) may be a scale wont to specify the acidity or basicity of the Associate in Nursing solution. Acidic solutions (solutions with higher concentrations of H+ ions) square measure measured to own lower pH values than basic or alcalescent solutions.
THE pH SCALE
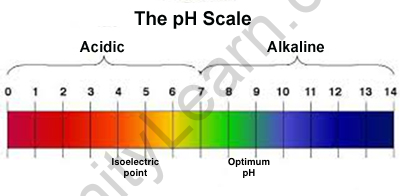
The hydrogen ion concentration is employed to rank solutions in terms of acidity or basicity (alkalinity). Since the dimensions rely on pH values, it’s an index, which means that an amendment of one pH unit corresponds to a ten-fold amendment in H^++start superscript, plus, finish superscript particle concentration. The hydrogen ion concentration is usually aforementioned to vary from zero to fourteen, and most solutions do fall at intervals this varies, though it’s attainable to induce a pH below zero or higher than fourteen. something below seven.0 is acidic, and something higher than seven.0 is alcalescent, or basic. The pH within human cells (6.8) and also the pH of blood (7.4) square measure each terribly near to neutral.
Extreme pH values, either higher than or below seven.0, square measure sometimes thought-about unfavourable for all times. However, the setting within your abdomen is very acidic, with a pH of one to a pair. however, will the abdomen get around this problem? The answer: disposable cells! abdomen cells, significantly those who are available in direct contact with abdomen acid and food, square measure perpetually dying and being replaced by new ones. In fact, the liner of the human abdomen is totally replaced within seven to 10 days.
Also read: Important Topic of Chemistry: Inorganic Compound
Most organisms, as well as humans, ought to maintain pH among a reasonably slim target in order to survive. for example, human blood must keep its pH right around seven.4, and avoid shifting considerably higher or lower – even though acidic or basic substances enter or leave the blood.
Buffers, solutions that will resist changes in pH, are key to maintaining stable H++ begin superscript, plus, finish superscript particle concentrations in biological systems. once there are too several H++ begin superscript, plus, finish superscriptions, a buffer can absorb a number of them, transferral pH back up; and once there are too few, a buffer can gift a number of its own H++ begin superscript, plus, finish superscriptions to scale back the pH. Buffers generally include associate acid-base combine, with the acid and base differing by the presence or absence of a nucleon (a conjugate acid-base pair).
Also read: Important Topic of Chemistry: Alkenes-Nomenclature
FAQs
Q. What solutions have a pH?
Ans. In general, pH values vary from zero to fourteen. The pH of a neutral resolution, i.e., one that is neither acidic nor base-forming, is 7. Acidic solutions have pH values below 7; base-forming, or basic, solutions have pH values higher than seven. A pH price provides a life of the proton concentration of an answer.
Q. How does one calculate the pH of a solution?
Ans. To calculate the particle concentration} of Associate in the Nursing solution you would like to understand the concentration of the hydronium ion in moles per litre (molarity). The pH is then calculated mistreatment the expression: pH = – log [H3O+].
Q. How are pH and resolution related?
Ans. As an answer gets additional basic (higher [OH–]), the pH will increase. because the pH of an answer decreases by one pH unit, the concentration of H+ will increase by 10 times. because the pH of an answer will increase by one pH unit, the concentration of OH– will increase by 10 times.
For more enhanced information on the subject, download the Infinity Learn app – the ultimate learning app for classes 3 to 13.





