Table of Contents
Acids and bases are famous chemical compounds that interact with every different, resulting in the formation of salt and water. The phrase acid comes from the Latin phrase ‘here’, which means ‘bitter’.
In our ordinary lives, we use many compounds that scientists name acids. The orange or grapefruit juice you drink for breakfast includes citric acid (additionally known as Vitamin C). When milk turns bitter, it consists of lactic acid. The vinegar used in salad dressing consists of acetic acid. According to this, a chemical bond is considered as being made up of an acid-base mixture. The properties of a molecule, consequently, can be understood by dividing it into acid and base fragments.
Acid Definition Chemistry
The terms acid and base have been defined in exceptional ways, relying on the unique manner of searching at the residences of acidity and basicity. Arrhenius first described acids as compounds that ionize to produce hydrogen ions and bases as compounds that ionize to supply hydroxide ions. According to the Lowry-Bronsted definition, an acid is a proton donor, and a base is a proton acceptor.
According to the Lewis definition, acids are molecules or ions capable of coordinating with unshared electron pairs, and bases are molecules or ions having unshared electron pairs available for sharing with acids. To be acidic inside the Lewis experience, a molecule has to be electron deficient. This is the maximum preferred acid-base concept. All Lowery Bronstead acids are Lewis acids, but, in addition, the Lewis definition includes many different reagents, which include boron trifluoride, aluminium chloride, and many others.
Theories of Acids and Bases
Three exclusive theories were placed forth to define acids and bases. These theories include the Arrhenius theory, the Bronsted-Lowry idea, and the Lewis concept of acids and bases. A quick description of each of those theories is provided in this subsection. Acids and bases may be described thru 3 unique theories.
The Arrhenius theory of acids and bases states that “an acid generates H+ ions in a solution while a base produces an OH– ion in its answer”.
The Bronsted-Lowry principle defines “an acid as a proton donor and a base as a proton acceptor”.
Finally, the Lewis definition of acids and bases describes “acids as electron-pair acceptors and bases as electron-pair donors”.
The pH of Acids and Bases
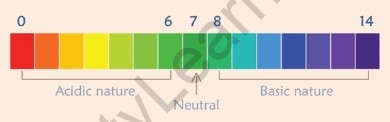
To discover the numeric fee of the extent of acidity or basicity of a substance, the pH scale (wherein pH stands for ‘potential of hydrogen) can be used. The pH scale is the maximum commonplace and trusted manner to a degree of how acidic or fundamental a substance is. A pH scale degree can range from 0 to fourteen, in which 0 is the maximum acidic and 14 is the maximum basic a substance can be.
Another manner of testing if a substance is acidic or basic is to use litmus paper. Two styles of litmus paper to be had may be used to become aware of acids and bases – crimson litmus paper and blue litmus paper. Blue litmus paper turns pink under acidic conditions, and red litmus paper turns blue under basic or alkaline conditions.
Properties of Acids and Bases
-
Properties of Acids:
Acids are corrosive.
- They are the right conductors of electricity.
- Their pH values are constantly much less than 7.
- When reacted with metals, these materials produce hydrogen gasoline.
- Acids are bitter-tasting substances.
Examples: Sulfuric acid [H2SO4], Hydrochloric acid [HCl], Acetic acid [CH3COOH].
-
Properties of Bases:
Some properties, like a bitter taste, are owned with the aid of all bases. The bases experience slippery, too. Dream on what slippery soap looks as if. And that is a basis. Furthermore, whilst immersed in water, bases conduct strength because they include charged particles within the solution.
- They are observed to have a soapy texture while touched.
- These materials launch hydroxide ions (OH– ions) whilst dissolved in water.
- In their aqueous answers, bases act as exact conductors of energy.
- The pH values corresponding to bases are continually extra than 7.
- Bases are sour-tasting materials that can turn purple litmus paper blue.
Examples: Sodium hydroxide [NaOH], milk of magnesia [Mg(OH)2], calcium hydroxide [Ca(OH)2].
-
Neutral Substances:
The neutral substance is a substance that has no acid or base residences, has an identical amount of hydrogen and hydroxyl ions, and does not modify the colouration of the litmus floor.
- These materials do not show any acidic or primary traits.
- Their pH values are approximately 7.
- Neutral substances do not affect red or blue litmus paper.
- The pH of pure water is exactly 7.
Examples: Water, Common salt (NaCl)
Uses of Acids and Bases
The numerous makes use of acids and bases are listed in this subsection.
Uses of Acids
- Vinegar, a diluted solution of acetic acid, has numerous household applications. It is commonly used as a meals preservative.
- Citric acid is a fundamental part of lemon juice and orange juice. It can also be used in the upkeep of meals.
- Sulfuric acid is broadly used in batteries. The batteries used to start the engines of vehicles usually contain this acid.
- The business production of explosives, dyes, paints, and fertilizers entails the usage of sulfuric acid and nitric acid.
- Phosphoric acid is a key ingredient in many gentle drinks.
Uses of Bases
- The production of cleaning soap and paper entails using sodium hydroxide. NaOH is also used in the manufacture of rayon.
- Ca(OH)2, also referred to as slaked lime or calcium hydroxide, is used to manufacture bleaching powder.
- Dry mixes utilized in portray or decoration are made with the help of calcium hydroxide.
- Magnesium hydroxide, additionally known as milk of magnesia, is typically used as a laxative. It also reduces any extra acidity in the human stomach and is, therefore, used as an antacid.
- Ammonium hydroxide is a completely critical reagent used in laboratories.
- Any excess acidity in soils may be neutralized via using slaked lime.
FAQs:
How do you identify acids and bases?
To decide if a substance is an acid or a base, before and after the reaction, be counted the hydrogens on each substance. If the number of hydrogens decreases, this product is the acid (which donates ions of hydrogen). If the range of hydrogens has risen, the substance is the foundation (accepts hydrogen ions).
What makes something acid or a base?
An acid is a contributing product containing hydrogen ions. Now the answer includes extra hydrogen ions than hydroxide ions. That form of solution is acidic. A foundation is a fabric capable of eating hydrogen ions as a base is dissolved in water, the equilibrium among hydrogen ions and hydroxide ions adjustments in the opposite direction.









