Table of Contents
An alkane is an acyclic saturated hydrocarbon in organic chemistry, also known as paraffin (a historical trivial name that also has other meanings). In other words, an alkane is made up of hydrogen and carbon atoms arranged in a tree structure with single carbon-carbon bonds. CnH2n+2 is the general chemical formula for alkanes. Alkanes range in complexity from the simplest case of methane (CH4) (often referred to as the parent molecule) to arbitrarily massive and complicated compounds like pentacontane (C50H102) or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane (C14H30). Alkanes are defined by the International Union of Pure and Applied Chemistry (IUPAC) as “acyclic branched or unbranched hydrocarbons with the general formula CnH2n+2, and thus consisting entirely of hydrogen atoms and saturated carbon atoms.” Despite having a distinct general formula, some sources use the term to refer to any saturated hydrocarbon, including those that are either monocyclic (i.e. cycloalkanes) or polycyclic (i.e. cycloalkanes are CnH2n). Each carbon atom in an alkane is sp3-hybridized with four sigma bonds (either C–C or C–H), and each hydrogen atom is joined to one of the carbon atoms (in a C–H bond). The carbon skeleton or carbon backbone of a molecule is the longest series of linked carbon atoms.
The number of carbon atoms can be thought of as the alkane’s size. One class of higher alkanes are waxes, which are solids at standard ambient temperature and pressure (SATP) and have more than 17 carbon atoms in their carbon backbone. The alkanes form a homologous series of organic compounds with repeated –CH2 units that differ in molecular mass by multiples of 14.03 u (the total mass of each such methylene-bridge unit, which consists of a single carbon atom of mass 12.01 u and two hydrogen atoms of mass 1.01 u each).
Methanogenic bacteria produce methane, and some long-chain alkanes act as pheromones in certain animal species or as protective waxes in plants and fungi. Nonetheless, most alkanes have little biological activity. They can be thought of as molecular trees from which the more active/reactive functional groups of biological molecules can be hung. Petroleum (crude oil) and natural gas are the two main commercial sources of alkanes. An alkyl group is a molecular fragment based on alkane that has one open valence for bonding. They are commonly abbreviated with the symbol for any organyl group, R, though Alk is occasionally used to specifically represent an alkyl group (as opposed to an alkenyl group or aryl group).
Overview
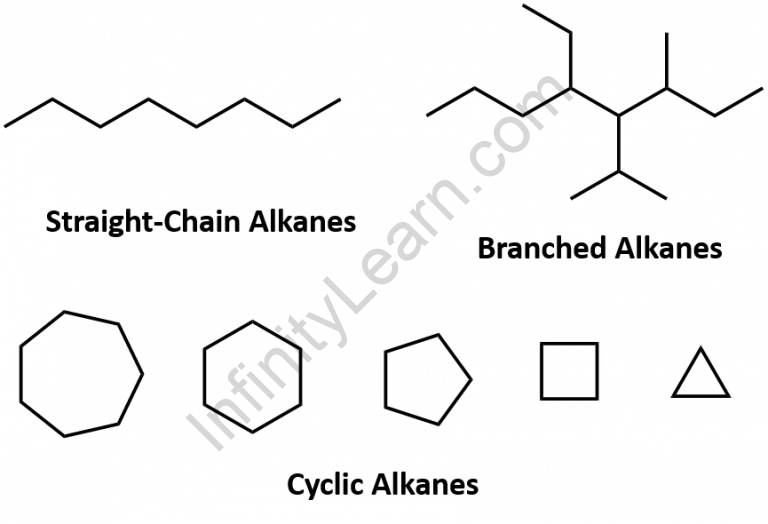
Alkanes are organic compounds made up entirely of single-bonded carbon and hydrogen atoms with no other functional groups. Alkanes have the general formula CnH2n+2 and are classified into three types: linear straight-chain alkanes, branched alkanes, and cycloalkanes. Alkanes are also saturated hydrocarbons, which means that all of the carbon atoms are’saturated’ with hydrogen atoms and have no carbon-carbon double or triple bonds. Alkanes are the least reactive and simplest hydrocarbon species, containing only carbons and hydrogens. They are commercially important because they are the primary constituents of gasoline and lubricating oils, and they are widely used in organic chemistry; however, the role of pure alkanes (such as hexanes) is primarily that of solvents. The absence of unsaturation distinguishes an alkane from other compounds that solely contain carbon and hydrogen. That is, it lacks double and triple bonds, which are extremely reactive in organic chemistry. Though they are not completely devoid of reactivity, their lack of reactivity under most laboratory conditions makes them a relatively uninteresting, albeit vital, component of organic chemistry. The energy confined within the carbon-carbon bond and the carbon-hydrogen bond is quite high, as you will learn later, and their rapid oxidation produces a large amount of heat, typically in the form of fire.
Alkane as saturated hydrocarbons
The most fundamental hydrocarbon family is the alkanes. They are entirely composed of carbon and hydrogen. Every carbon atom has four bonds, while every hydrogen atom only has one. Line-angle formulas are preferred by chemists over condensed structural formulas because they are easier and faster to draw. Condensed alkane structural formulas are also available.
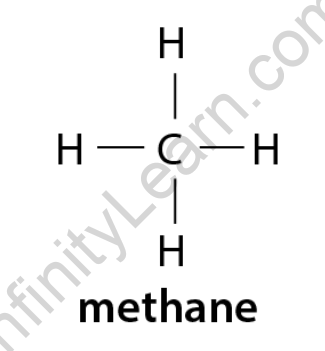
Methane, with the chemical formula CH4, is a simple alkane with one carbon atom. This molecule’s structural formula is because it only has one covalent bond.
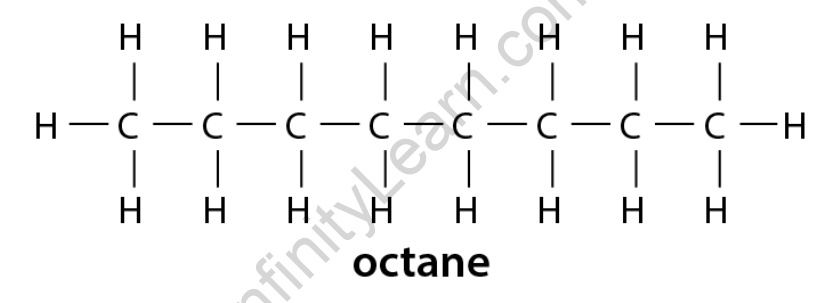
A single covalent bond connects additional carbon atoms in a long chain alkane molecule. Each atom is joined to four hydrogen atoms to form four single covalent bonds. This long-chain structure is known as octane. An eight-carbon alkane has the structural formula C 8H 18 and the molecular formula C 8H 18.
Physical Properties of Alkanes
The Alkane Solubility
Alkanes are generally non-polar molecules due to the small difference in electronegativity between carbon and hydrogen and the covalent nature of the C-C or C-H bond. Polar molecules, as we know, are soluble in polar solvents, whereas non-polar molecules are soluble in non-polar solvents. As a result, alkanes are hydrophobic in nature, which means they are insoluble in water. They are soluble in organic solvents, however, because the energy required to overcome existing Van Der Waals forces and generate new Van Der Waals forces is comparable.
The Alkanes’ Boiling Point
As the intermolecular Van Der Waals forces increase with the molecule’s molecular size or surface area, we observe: The boiling point of alkanes increases with increasing molecular weight, with straight-chain alkanes having a higher boiling point than structural isomers.
The Alkane Melting Point
The melting point of alkanes follows the same trend as their boiling point, i.e. it rises as molecular weight rises. This is because higher alkanes are solids, making it difficult to overcome intermolecular forces of attraction between them. Even-numbered alkanes have a higher melting point trend than odd-numbered alkanes because the even-numbered alkanes pack well in the solid phase, forming a well-organized structure that is difficult to break.
Alkane Formula Chemistry
Organic compound formulas present information at various levels of sophistication. Molecular formulas, such as that of octane, specify the number of each type of atom in a compound’s molecule. The molecular formula C8H18 can refer to a variety of alkanes, each with its own set of chemical, physical, and toxicological properties. These various compounds are identified by structural formulas that show the arrangement of the atoms in a molecule. Compounds with the same molecular formula but different structural formulas are referred to as structural isomers. Alkanes are the building blocks for the majority of organic compounds.
Furthermore, many important parts of organic molecules contain one or more alkane groups bonded as substituents onto the basic organic molecule, minus a hydrogen atom. As a result of these factors, many organic compound names are based on alkanes.
FAQ’s
Which of the following are the first four alkanes?
The first four alkanes are methane (CH4), ethane (C2H6), propane (C3H8), and butane (C4H10). The simplest alkane is methane gas, which has the molecular formula CH4.
What is the classification of alkanes?
Alkanes are hydrocarbon atoms with a single bond. There are three types of alkanes: linear straight alkanes, branched alkanes, and cyclic alkanes.
Is alkane classified as a functional group?
Alkanes are not typically thought of as functional groups; rather, an alkane is a compound that lacks functional groups. A functional group in an alkene is a carbon-carbon double bond.






