Table of Contents
Introduction:
To balance chemical equations, stoichiometric coefficients are added to the reactants and products. This is significant because a chemical equation must respect the laws of conservation of mass and constant proportions, which require that the same amount of atoms of each element exist on the reactant and product sides of the equation.
Chemical Equation
A chemical equation is a symbolic description of a chemical process in which the reactants and products are represented by chemical equations.
2H2 + O2 →2H2O is an example of a chemical equation that describes the reaction between hydrogen and oxygen to generate water.
The reactant side of the chemical equation is to the left of the → sign, while the product side is to the right of the arrow symbol.
Coefficient of Stoichiometry
The total number of molecules of a chemical species that participate in a chemical reaction is described by a stoichiometric coefficient. It gives a ratio of the responding species to the products of the reaction.
The stoichiometric coefficient of O2 and H2O in the reaction given by the equation CH4 + 2O2 →CO2 + 2H2O is 2, whereas that of CH4 and CO2 is 1.
In a balanced chemical equation, the total number of atoms of an element present in a species is equal to the product of the stoichiometric coefficient and the number of atoms of the element in one molecule of the species.
The total number of oxygen atoms in the reactive species ‘2O2‘, for example, is four.
Stoichiometric coefficients are assigned in a way that balances the total number of atoms of an element on the reactant and product sides when balancing chemical equations.
Follow these four basic steps to balance a chemical equation:
- Write the unbalanced equation to indicate the reactants and products.
- Determine how many atoms of each element are on either side of the reaction arrow.
- Multiply coefficients to equalize the number of atoms of each element on both sides of the equation (the numbers in front of the formulas). The atoms of hydrogen and oxygen are the simplest to balance last.
- Indicating the state of matter of the reactants and products can help you check your work.
The Traditional Method of Balancing
The first step in balancing chemical equations is to get the imbalanced equation in its entirety. The combustion process between propane and oxygen is used as an example to demonstrate this approach.
Step 1: Determine the unbalanced equation using the chemical formulas of the reactants and products (if it is not already provided).
C3H8 is the chemical formula for propane. When it reacts with oxygen (O2), it produces carbon dioxide (CO2) and water (H2O)
The imbalanced chemical equation is C3H8 + O2 →CO2 + H2O.
Step 2: Compare the total number of atoms of each element on the reactant and product sides.
Step 3: Stoichiometric coefficients are now applied to molecules that contain an element with a varied number of atoms on the reactant and product sides.
The number of atoms on either side must be balanced by the coefficient. In general, the stoichiometric coefficients for hydrogen and oxygen atoms are assigned last. The number of atoms in the reactant and product elements must now be changed. It should be noted that the number of atoms of an element in one species must be calculated by multiplying the stoichiometric coefficient by the total number of atoms of that element present in one molecule of the species.
Step 4: Step 3 is continued until the number of atoms of the interacting elements on the reactant and product sides is equal. Hydrogen is balanced next in this scenario. The following is a transformation of the chemical equation.
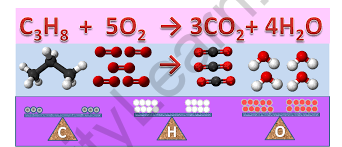
C3H8 + O2 → 3CO2 + 4H2O
Now that the hydrogen atoms have been balanced, the oxygen atoms must be balanced. Because the product side has 10 oxygen atoms, the reactant side must likewise include 10 oxygen atoms.
Each O2 molecule has two oxygen atoms. As a result, the O2 molecule must be allocated a stoichiometric value of 5. The corrected chemical equation may be found below.
C3H8 + 5O2 → 3CO2 + 4H2O
Step 5: After all of the individual components have been balanced, the total number of atoms of each element on the reactant and product sides is compared again.
The chemical equation is considered to be balanced if there are no inequalities.
In this case, each element now has an equal amount of atoms on both the reactant and product sides.
The balanced chemical equation is, therefore, C3H8 + 5O2 → 3CO2 + 4H2O.
FAQ’s
Q. What is the chemical equation for the balanced reaction of ferric chloride and sodium hydroxide?
ANS: Ferric chloride has the chemical formula FeCl3 while sodium hydroxide has the chemical formula NaOH. The imbalanced chemical equation is as follows:
Fe(OH)3 + NaCl →Fe(OH)3 + NaCl
The balanced chemical equation is determined by first balancing the amount of oxygen and hydrogen atoms and then balancing the number of sodium atoms:
Fe(OH)3 + 3NaCl→ Fe(OH)3 + 3NaCl
Q. Can you provide some helpful hints for balancing a chemical equation?
ANS: When balancing equations, keep in mind that chemical processes must adhere to the principle of mass conservation. Check your work to make sure the number and kind of atoms on the reactants and products sides are the same. A chemical’s atoms are multiplied by a coefficient (number in front of it). Only the number of atoms immediately after a subscript (the lower number) is doubled. If there is no coefficient or subscript, it is the same as a number “1.” (which is not written in chemical formulas).
Q. What is a balanced chemical equation? What is the purpose of balancing chemical equations?
ANS: A balanced chemical reaction is defined as an equation with equal numbers of each type of atom on both sides of the arrow.
A chemical equation is a written symbolic representation of a chemical process. The reactant(s) are listed on the left, while the product(s) are listed on the right. Due to the fact that atoms cannot be created or destroyed during a chemical reaction, the number of atoms present in the reactants must match the number of atoms present in the products, according to the rule of conservation of mass.
Q. What is the mass conservation law?
ANS: According to the rule of conservation of mass, in a chemical reaction, the reactants react with one another to form products, but the total mass of the reactants before and after the chemical change is the same.
In other words, the law of conservation of mass stipulates that the total mass of chemical species remains constant during a chemical process.