Table of Contents
Bohr’s Theory Of Hydrogen Atoms
Bohr model of the hydrogen atom was the first atomic model to effectively make sense of the radiation spectra of atomic hydrogen. Niels Bohr presented the atomic Hydrogen model in the year 1913. Bohr Model of the hydrogen atom endeavours to connect specific holes as proposed by Rutherford’s model. It holds a unique spot in history as it led to quantum mechanics by presenting the quantum hypothesis.
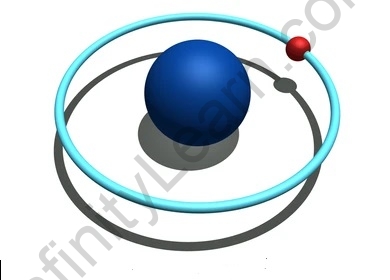
Planetary Model of the Atom
- Quantum mechanics arose during the 1920s. Neil Bohr, one of the authors of quantum mechanics, was keen on the much-discussed subject of the time – the construction of the atom. Various atomic models, including the hypothesis proposed by J.J Thompson and the disclosure of core by Ernest Rutherford, had arisen. In any case, Bohr upheld the planetary model, which affirmed that electrons rotated around a decidedly charged core very much like the planets around the sun.
All things considered, researchers actually had numerous unanswered inquiries, for example,
- For what reason didn’t the electrons drop into the core as predicted by old-style material science?
- Where are the electrons and what do they do there?
- How is the discrete emanation lines delivered by invigorated components connected to the interior construction of the atom?
Bohr resolved this large number of inquiries utilizing an apparently basic suspicion: What assuming electron circles and energies, could display just explicit qualities? You can actually look at Atomic Theory to find out about the different atomic hypotheses set forward by researchers in the mid-twentieth century.
Energy Level – Principle Energy Level
Bohr’s Atomic Model:
- Thomson’s and Rutherford’s atomic models neglected to address any inquiries connected with the energy of an atom and its solidness. In the year 1913, Niels Bohr proposed an atomic model, portraying an atom as a little, emphatically charged core encompassed by electrons that movement in roundabout circles around the decidedly charged core, like the planets around the sun in our planetary group, with fascination given by the electrostatic powers.
- This model is prominently known as the Bohr model of an atom. Bohr proposed an atomic model of a hydrogen atom.
- Bohr’s model gave a legitimate clarification for the solidness of electrons spinning in circles. He named these circles as energy shells.
Bohr’s Equation
- Bohr Model of the hydrogen atom first proposed the planetary model, yet later a presumption concerning the electrons was made. The supposition that was the quantization of the design of atoms. Bohr’s suggested that electrons circled the core in explicit circles or shells with a decent span. Just those shells with a range given by the situation beneath were permitted, and it was unimaginable for electrons to exist between these shells.
- Numerically, the permitted worth of the atomic range is given by the situation:
r(n)=n2 x r(1)
Where,
- n is a positive whole number
- r(1) is the littlest permitted sweep for the hydrogen atom otherwise called the Bohr’s range
The Bohr’s radius has a value of r(1)=0.529 x 10-10m
Bohr determined the energy of an electron in the nth degree of hydrogen by considering the electrons in a roundabout, quantized circles as:
E(n)=-1/n2 x 13.6eV
Where,
- 13.6 eV is the most minimal conceivable energy of a hydrogen electron E(1).
- The energy acquired is generally a negative number and the ground state n = 1, has the most bad worth. The explanation being that the energy of an electron in circle is comparative with the energy of an electron that is completely isolated from its core, and it is perceived to have an energy of 0 eV. Since the electron in a decent circle around the core is more steady than an electron that is incredibly a long way from its core, the energy of the electron in circle is negative all the time.
Absorption and Emission
- As indicated by Bohr’s model, an electron would ingest energy as photons to become eager to a higher energy level. Subsequent to running away to the higher energy level, otherwise called the invigorated express, the energized electron is less steady, and along these lines, would quickly emanate a photon to return to a slower, more steady energy level. The energy of the transmitted photon is equivalent to the distinction in energy between the two energy levels for particular progress. The energy can be determined utilizing the condition:
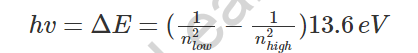
Limitations of the Bohr Model of the Hydrogen Atom:
- Bohr’s model doesn’t function admirably for complex atoms.
- It couldn’t make sense of why a few otherworldly lines are more extraordinary than others.
- It couldn’t make sense of why a few otherworldly lines split into various lines within the sight of an attractive field.
The Heisenberg’s vulnerability standard goes against actual electrons for Bohr existing in explicit circles with a known sweep and speed.
Albeit the advanced quantum mechanical model and the Bohr Model of the Hydrogen Atom might appear to be immeasurably changed, the key thought is something very similar in both. Traditional material science isn’t adequate to depict every one of the peculiarities that happen on an atomic level. Yet, Bohr was the first to understand the quantization of electronic shells by melding the possibility of quantization into the electronic design of the hydrogen atom and was effectively ready to make sense of the discharge spectra of hydrogen as well as other one-electron frameworks.
Summary:
- Bohr recommended that electrons travel in explicit circles, shells around the core.
As indicated by Bohr’s computation, the energy for an electron in the shell is given by the articulation: - E(n)= -1/n2 x 13.6eV
- The hydrogen range is made sense of as far as electrons retaining and discharging photons to change energy levels, where the photon energy is:

- Bohr’s Model of the Hydrogen Atom isn’t pertinent for frameworks with more than one electron.
FAQs
Question: State two limitations of Bohr’s model of the atom.
Answer:
- It infringes upon the Heisenberg Uncertainty Principle.
- The Bohr Model considers electrons to have both a known radius and orbit, which is unimaginable, as per Heisenberg.








