Table of Contents
Bronsted Lowry Theory
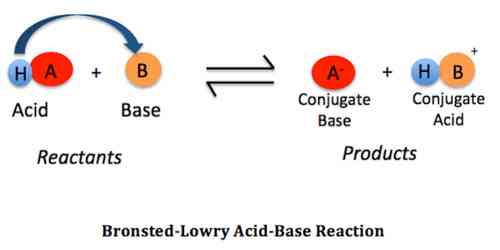
According to the Bronsted-Lowry system, an object can act as an acid only where there is a base; similarly, an object can only serve as a base where there is acid. In addition, when an acidic substance loses its proton, it forms a base, called the conjugate acid-base, and when the base gains a proton, it forms an acid called the conjugate acid-base. Therefore, the reaction is between an acidic substance, such as hydrochloric acid, and a basic substance.
Bronsted-Lowry theory increases the number of compounds considered acids and bases so that they include not only neutral molecules (e.g., sulfuric, nitric, acetic acid, and alkali metal hydroxides) but also certain atoms and -molecule positive and negative. electric charges (cations and anions). Ammonium ions, hydronium ions, and other hydrated metals are considered acids. Acetate, phosphate, carbonate, sulphide, and halogen ions are considered bases.
The strong acid is a type that completely separates its ions from an aqueous solution. Nitric acid is an example of a strong acid. It completely dissolves in water to form hydronium, and nitrate ions. After the reaction occurs, there are no uninsulated molecules in the solution.
In contrast, a weak acid does not completely separate from its ions. An example of a weak acid is acetic acid, which is present in vinegar. Acetic acid dissolves slightly in water to form hydronium and acetate ions.
Bronsted-Lowry Acid:
Bronsted-Lowry acid is a potential source of protons. For example, HCl is Bronsted-Lowry acid as it has the ability to donate protein. When Bronsted-Lowry acid contributes to the proton, a base is formed, and this base is called the conjugate base. For example, the conjugate base of the HCl molecule is ion chloride.
Bronsted-Lowry Base:
The Bronsted-Lowry base is a proton-absorbing material. For example, NH3 is a Bronsted-Lowry base as it has the ability to receive protons. When the Bronsted-Lowry base adopts a proton, an acid is formed, and this acid is called conjugate acid. For example, the conjugate acid molecule of NH3 is a cation of ammonium
Bronsted-Lowry Acid-Base Reaction:
The Brønsted-Lowry acid-base reaction involves the transfer of protons from the acid to the base. In this process, after donating a proton, the first acid becomes the basis of the conjugate, and after receiving the proton, the first base becomes conjugate acid. Thus, any Bronsted-Lowry acid-base reaction combines two acids with two bases, forming a pair of conjugate acid-base.
Key Points of the Bronsted Lowry Theory:
- Bronsted-Lowry acid is a type of chemical that can provide proton or hydrogen cation.
- The Bronsted-Lowry base is a type of chemical that is able to absorb protons. In other words, it is a type with a single pair of electrons available to be synthesised on H +.
- After Bronsted-Lowry acid donates protein, it forms its own conjugate base. Conjugate acid-base of Bronsted-Lowry forms when it receives a proton. A pair of conjugate acid-base has a molecular formula similar to the original pair of acid-base, except that the acid has one more H + compared to the conjugate base.
- Solid acids and bases are defined as compounds that add ionise completely to water or an aqueous solution. Acids and weak bases are slightly separated.
- According to this theory, water is amphoteric and can serve as a base for Bronsted-Lowry acid and base.
When asked to indicate whether a chemical reaction includes solid acids or bases or weak ones, it helps to look at the arrow between reactants and products. The solid acid or base separates completely from its ions, leaving no ions uncoated after the reaction has been eliminated. The arrow usually points from left to right.
On the other hand, weak acids and bases do not completely separate, so the reaction arrow points both left and right. This reflects the dynamic balance that is established when a weak acid or base and its fragmented form both remain in solution.
Conjugate Acids-Bases:
When a substance acting as Bronsted-Lowry acid delivers its proton, it becomes the basis for the opposite reaction. In the above reaction, the hydrogen sulphate ion contributes a proton to water and becomes a sulphate ion. HSO 4 – and SO 4 2− are connected to each other by the presence or absence of H + ions. A conjugate acid-base pair is a pair of substances associated with the loss or gain of a single hydrogen ion. Conjugate acid is a particle produced when the base receives a proton. The hydrogen sulphate ion is the conjugate acid of ion sulphate. The basis of the conjugate is the particles produced when the acid contributes to the proton. The sulphate ion is the basis of the hydrogen sulphate ion conjugate.
FAQs
What is the difference between Bronsted-Lowry theory and Lewis theory?
Bronsted-Lowry's theory is based on proton transfer, Lewis's theory is based on electron transfer. Lewis acid is a substance that absorbs a pair of electrons to form a new bond. They are sometimes called electrophiles, or those who want an extra electron pair.
Why are Bronsted definitions so useful?
Bronsted-Lowry acid-base theory has several advantages over Arrhenius theory: for example, only Bronsted theory describes the reaction between acetic acid and ammonia, which do not produce hydrogen ions in solution. Water is amphoteric, which means it can act as an acid or base.
What is the difference between Bronsted acid and Bronsted base?
In the Bronsted – Lowry definition of acids and bases, the acid is the proton (H⁺) supplier, and the base is the proton receptor. When Bronsted-Lowry acid loses protein, a conjugate base is formed. Similarly, when the Bronsted-Lowry base acquired protons, conjugate acid formed.
How do you see the base of Bronsted-Lowry?
To determine if an item is acidic or basic, calculate the hydrogen in each item before and after the reaction. When the amount of hydrogen is reduced then the acid releases hydrogen ions. When the amount of hydrogen is increased, the base receives hydrogen ions.
Does the Bronsted-Lowry base provide electrons?
If the bond between Bronsted-Lowry acid and proton breaks, Bronsted-Lowry acid provides the proton. Thus, Lewis's base can donate electrons to the proton, while Bronsted-Lowry acid donates that proton.








