Table of Contents
Introduction
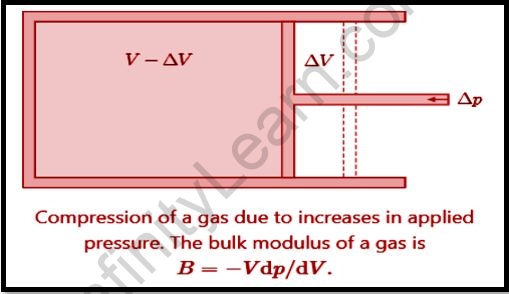
The Bulk Modulus is attributed to the difference in the volume of a body generated by a uniformly applied unit compressive or tensile stress throughout the surface.
A brief outline
The bulk modulus is described as the proportion of volumetric stress which can influence the volumetric strain of a certain material when the material displacement would be within the elastic limit. To put it differently, the bulk modulus is a numerical constant that is being used to quantify and explain the elastic properties of a solid or fluid when all surfaces are subjected to pressure.
Important concepts
Every gas has an equation that links density, pressure, and temperature, known as the state equation. This allows us to derive an explicit density-pressure connection. Such correlations are predicated on the type of process being studied, such as isentropic at constant entropy or isothermal at a constant temperature. The inferred equations are written in the following format.
p/ho = constant for the isothermal process – (1)
And
p/hok = constant for the isentropic process – (2)
Where k signifies the ratio of specific heat at constant pressure cp to the specific heat at persistent volume cv. Furthermore, cp – cv = R where R directs gas constant. Under normal situations, k = 1.4 for air.
When you replace equations 1 and 2 in the bulk modulus definition, we get,
Ev = p for isothermal process
Ev = kp for isentropic process
As a consequence, the bulk modulus of a gas is determined by its pressure. At STP, the ambient pressure is 1.01325 x 105N/m2, and the bulk modulus is of comparable order, at 2.15 x 109 N/m2. Air is over 15,000 times more compressible than water, according to these figures.
Significance of bulk modulus gases in IIT JEE exam
Solids and liquids properties, which account for 3 to 4% of exam questions, encompass a wide range of topics. All you have to do is grasp the concepts of each topic. Based on the distribution of difficulty levels in previous years’ tests, we should expect 10 to 12 marks beneath the topic.
FAQs
What is the relationship between compressibility and bulk modulus?
Compressibility and bulk modulus are known to be connected in physics. These two concepts primarily deal with the ideas of pressure and volume. It's crucial to grasp what compressibility and bulk modulus mean before diving into the relationship between the two.
What does it imply to say that something is compressible?
Compressibility refers to the ability to be compressed into a smaller space by applying pressure. The compressibility of a fluid refers to the amount of density change that a given change in pressure causes in the fluid. Gases, on the other hand, are squeezable to a large extent, but most liquids are not.
Q. Dimensional Formula for Bulk Modulus?
Ans: The dimensional formula for bulk modulus is as follows:
[M1 L-1 T-2]