Table of Contents
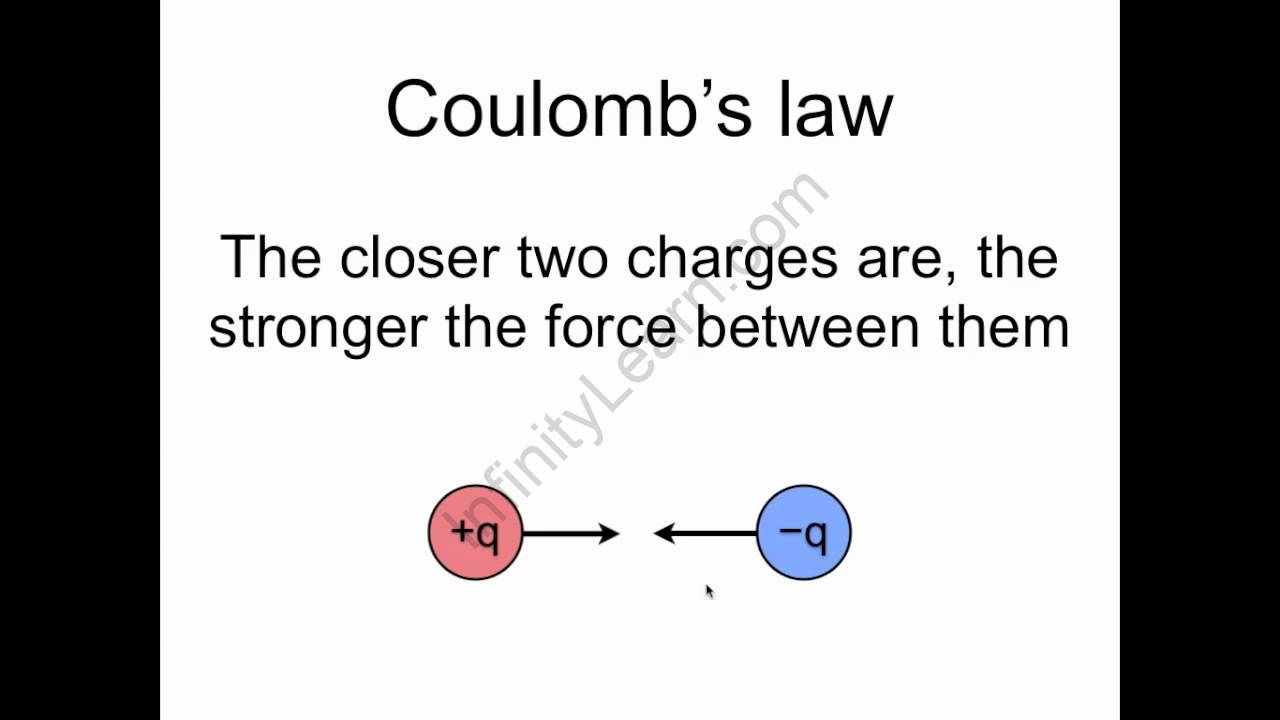
Coulomb’s Law
Coulomb’s law is a mathematical description of the electric force that exists between charged objects. It is analogous to Isaac Newton’s law of gravity and was developed by the 18th-century French physicist Charles-Augustin de Coulomb. Gravitational and electric forces both decrease with the square of the distance between the objects and act along a line that connects them. Coulomb’s law, on the other hand, uses an object’s electric charge rather than its mass to determine the magnitude and sign of its electric force.
As a result, charge determines how electromagnetism affects charged object motion.
The fundamental property of matter in charge. Every component of matter has an electric charge, which can be positive, negative, or zero. For example, electrons are negatively charged, whereas atomic nuclei are positively charged. The net charge of most bulk matter is zero because it has an equal amount of positive and negative charges.
According to Coulomb’s law, the force of attraction or repulsion between two charged bodies is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. It operates along the line that connects the two point charges.
Coulomb’s Law Formula
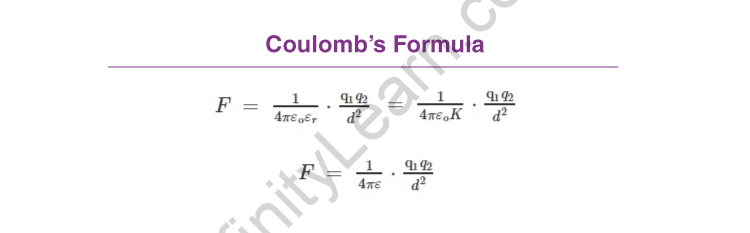
F ∝ q1q2/d2
ε is the absolute permittivity, K or εr is the relative permittivity, and the specific inductive capacity.
ε0 represents the permittivity of free space.
The dielectric constant of the medium in which the two charges are placed is also known as K or εr.
What is Coulomb of Charge?
When two charges are separated by one metre in a vacuum, a coulomb is defined as the charge that repels an equal charge of the same sign with a force of 9×109 N. Coulomb force is a conservative mutual and internal force. εo has a value of 8.86 × 10-12 C2/Nm2 (or) 8.86 × 10-12 Fm–1
Coulomb’s Law – Conditions for Stability
When q is slightly shifted towards A, FA increases in magnitude while FB decreases. Because the net force on q is now directed toward A, it will not return to its original position. As a result, the axial displacement equilibrium is unstable. When q is moved perpendicular to AB, the forces FA and FB return the charge to its original position. As a result, the equilibrium is stable for perpendicular displacement.
Limitations of Coulomb’s Law
- Only point charges at rest are covered by the law.
- Coulomb’s Law applies only when the inverse square law is followed.
- When charges have an arbitrary shape, it is difficult to apply Coulomb’s law because we cannot determine the distance’ between them.
- The law cannot be used to calculate the charge on the large planets directly.
FAQs:
What is the significance of Coulomb's law?
Coulomb's law denotes electric force's inverse square dependence. The law is also used accurately in the derivations of Gauss' law for general cases. Coulomb's law in vector form is important because it specifies the direction of electric fields caused by charges.
Where does Coulomb's law come into play?
Coulomb's Law has numerous applications in modern life, including Xerox machines, laser printers, and powder coating. The ancient peoples who lived around the Mediterranean Sea knew that rubbing an amber rod on a cat's fur would attract light objects such as feathers.







