Table of Contents
Elements having electrons (1 to 10) are within the d-orbital of the penultimate power stage, and in the outermost ‘s’ orbital (1-2) are d-block elements. Although electrons no longer top off ‘d’ orbital in the group 12 metals, their chemistry is similar in lots of approaches to that of the previous corporations, and so considered as d block factors.
These elements typically show metallic features, malleability and ductility, high electrical and thermal conductivity values, and proper tensile electricity. There are four collections within the d block, similar to the filling up of 3d, 4d, 5d, or 6d orbitals.
3d- Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn
4d- Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd
5d- La, Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg
6d- incomplete.
10 elements fill up the ‘d’ orbital in each series.
D block factors occupy columns three to 12 and might have atoms of factors with completely stuffed ‘d’ orbital. IUPAC defines a transition metal as “a detail whose atom or cations has a partially stuffed d sub-shell.
Why are D Block Elements known as Transition Elements?
Transition elements occupy businesses 4–11. Scandium and yttrium of group 3, having a partially filled d subshell within the steel state, are also considered transition elements. Elements like Zn, Cd, and Hg of the 12 columns of the d block have crammed d-orbital and, as a result, aren’t considered transition factors.
Transition Elements are so named, indicating their positioning and transition of properties among s and p block factors. So, all transition metals are d-block elements, but all d-block elements are not transitioned factors.
Properties of Transition Metals
Electrons are added to the ‘d’ sub-orbitals that lie among their (n+1) s and (n+1) p sub-orbitals.
It is placed between s and p block factors inside the periodic table.
Properties between s and p-block factors.
Electronic Configuration of D Block Elements
D block Elements have a general electronic configuration of (n-1)d 1-10ns 1-2. These factors can locate stability in half-stuffed orbitals and crammed d orbitals. An instance of this will be the electronic configuration of chromium, which has 1/2-stuffed d and s orbitals in its configuration – 3d54s1. The electronic configuration of copper is any other such instance. Copper shows a digital configuration of 3d104s1 and no longer 3d94s2.
This can be attributed to the relative stability of the stuffed d orbital. Zinc, Mercury, Cadmium, and Copernicium showcase completely stuffed orbitals in their floor states and well-known oxidation states. Those metals aren’t considered transition factors for this motive, whereas the others are d-block elements.
- The digital configuration for length 4, transition factors is (Ar) 4s 1-2 3d 1-10
- The digital configuration for period five, transition elements is (Kr) 5s 1-2 4d 1-10
- The digital configuration for period 6, transition elements is (Xe) 4s 1-2 3d 1-10
Along with the duration, from left to proper, electrons are introduced to the 3d subshell as in step with the Aufbau precept and Hund’s rule of multiplicity.
Anomalies occur in all of the series, which can be defined from the subsequent concerns.
The strength hole among the ns and (n-1) d orbitals
Pairing strength for the electrons in s-orbital
Stability of half-crammed orbitals to partially crammed orbitals.
Chromium has 4s13d5 electron configuration instead of the 4s23d4 configuration and copper 4s13d10 in preference to 4s23d9. These anomalies within the first transition series may be understood from the stability of 1/2-filled orbitals compared to the partially crammed orbitals.
In the second collection of transition metals from niobium, electron presence in d orbitals appears to be desired rather than shared in s orbitals. Between the available s and d orbitals, the electron can both go for sharing in the s-orbital or excited to the d-orbital. The selection relies upon the repulsive electricity it has triumphed over on sharing and the strength hole among the s and d-orbitals.
In the second collection, s and d-orbital have almost identical power because electrons favor occupying the d-orbital. So from niobium, s-orbital has mainly only one electron. Third-series transition metals, however, have greater paired-s configurations even at the price of half-filled orbitals (Tungsten- 6s25d4). This series comes after the filling up of 4f orbitals and resulting lanthanide contraction.
The decreased length affects the excessive protection of d orbitals by means of the ‘f’ electron. This protection increases the electricity gap between the s and 5d orbitals, so pairing electricity is less than the excitation. Excitation of the electron no longer takes the vicinity in tungsten, no matter the stableness possible, because of 1/2-filled orbitals.
Atomic and ionic radii of factors of all three-transition collection
- Decreases hastily, from column 3 to six
- Remains steady from columns 7 to 10 and
- Starts growing from columns 11 to twelve.
For example, within the first transition collection, atomic radii, the decrease from Sc to Cr (institution three to 6 ) is nearly the same for Mn, Fe, Co, and Ni (groups 7,8, nine &10), and growth in Cu and Zn.
The large lower in atomic radii, in columns 3 to 6 elements, is because of the increase in effective nuclear rate; however poor protection due to the smaller wide variety of d-electrons.
In elements of columns 7 to 10, growing powerful nuclear charge is balanced with the aid of the repulsion between the shared d electrons in order that radii remain identical.
In the case of 11 and 12-column elements, the d orbital is complete with ten electrons and protects the electrons in the better s-orbital. So, companies 11 and 12 factors like Cu and Zn have larger sizes than their in advance elements in the block.
Since electrons occupy a better orbital, the radii of the 0.33 collection are more than the second series factors. But the radii of each collection are almost the same. In the third series of factors, 5d orbitals are filled handiest after the filling up of 4f orbitals, which will increase the powerful nuclear rate by means of 14 gadgets.
This better nuclear rate ends in the larger shrinkage of radii known as Lanthanide contraction. A boom in radii because of the higher orbital might be successfully neutralized by the increase in the nuclear power price. So, the radii of the 2nd and 1/3 series elements have equal atomic radii. For example, Niobium and hafnium have nearly equal atomic radii.
Properties of D Block Elements
Ionization Energy of D Block Elements
Ionization strength is the energy needed to get rid of the valence electron from the atom/ion and is directly associated with the force of enchantment at the electron. Hence, the larger the nuclear price and the smaller the electron radii might be the ionization power (IE). Ionization Energy also could be greater for 1/2-stuffed and crammed orbitals.
The Ionization Energy of the d-block factors is greater than the s-block and smaller than the p-block elements, among which they may be located. In the first collection, except for chromium and copper, the first Ionization Energy includes removal from crammed s-orbital. Among them, the ionization power of d block factors will increase with the growth in the atomic range up to Fe.
In Co and Ni, increasing sharing of d-electrons makes amends for the atomic variety boom ensuing in the lower stages of Ionization Energy. Copper and zinc show increasing IE as s -block elements. In the second collection, elements from Niobium have single electrons inside the s-orbital.
Hence, they display a gradual increase in IE with increasing atomic numbers. Palladium, on the other hand, has a completed-shell and no electron in the s-shell. So, Pd suggests the most IE. Because of lanthanide contraction, the appeal of electrons by using the nuclear charge is much better, and subsequently, IE of 5d factors are a great deal larger than 4d and 3d. In the 5d series, all elements except Pt and Au have filled the s-shell.
Elements from Hafnium to rhenium have the same IE, and after IE increases with the number of shared d-electrons, Iridium and Gold have the maximum IE.
Metallic Character
D block factors show traditional metallic behaviour of excessive tensile power, malleability, ductility, electrical and thermal conductivity, metal luster, and crystallization in bcc/ccp/hcp systems.
They are very difficult and feature high enthalpy of atomization and occasional volatility, except for Copper. Hardness will increase with the range of unpaired electrons. Hence Cr, Mo, and W are very hard metals amongst d block factors. The group-12 factors (Zn, Cd, and Hg) also show the exception.
Oxidation States of D Block Elements
An oxidation country is a hypothetical country wherein the atom appears to launch or gain electrons extra than the standard valency kingdom. It continues to be beneficial in explaining the homes of the atom/ion. Transition factors/ions may have electrons in both s and d-orbitals.
Since the energy difference among s and d-orbital are small, each of the electrons can involve in ionic and covalent bond formation and hence showcase a couple of(variable) valency states (oxidation states).
Each transition element can subsequently showcase a minimal oxidation nation similar to the wide variety of s-electrons and most oxidation kingdoms equivalent to the total range of electrons available in each s and d-orbitals. In between oxidation states additionally become feasible.
Trends inside the Oxidation States
- The minimum Oxidation kingdom of 1 is shown using Cr, Cu, Ag, Au, and Hg.
- More solid Oxidation kingdoms will increase within the order 3d ˂ 4d ˂, 5d. 3d collection elements are maximum solid in +2, 4d series in +2 and +four, and 5d series in +four. Cr6+ and Mn7+ (of 3d) are not stable in their better OS. Compounds containing them, CrO42- and MnO4– are very reactive and sturdy oxidizing agents.
While Mo6+ and Tc7+ (of 4d) are stable with their better OS., the Compounds containing them, MoO42- and TcO4–, are unreactive and stable. Similarly, W6+ and Re7+ (of 5d) are strong in their better OS. Compounds containing them, WO42- and ReO4– are unreactive and strong.
Cations of the second one and 0.33-row transition metals in decreased oxidation states (+2 and +three) are a good deal greater effortlessly oxidized than the corresponding ions of the primary-row transition metals. For instance, the maximum solid compounds of chromium are the ones of Cr(III). However, the corresponding Mo(III) and W(III) compounds are reactive.
In reality, they may be frequently pyrophoric, bursting into flames on contact with atmospheric oxygen. As we will see, the heavier elements in every group form strong compounds in higher oxidation states that have no analogs with the lightest member of the organization.
Three. Strongly oxidizing, high oxidation range elements shape compounds of oxides and fluorides and not bromides and iodides.
Vanadium shape best VO4–, CrO42-, MnO4–, VF5, VCl5, VBr3, VI3, and now not VBr5, VI5. V5+ oxidizes Br– and I– to Br2 and I2; however, not fluoride because of its high electronegativity and small length.
Similarly, strongly reducing, low oxidation quantity factors form bromides and iodides, not oxides and fluorides.
Four. The maximum oxidation state, the same as the s and d-electrons, is exhibited using center-order factors in each series. Thus, manganese in the 3d series has +7, Ru in 4d, and Os in 5d own +eight most oxidation kingdoms.
- Elements may show all the Oxidation states are among the minimal and most.
- Elements in their decrease oxidation states can be ionic and simple (TiO, VO, CrO, MnO, TiCl2, and VCl2) in-between country amphoteric (Ti2O3, V2O3, Mn2O3, CrO3, Cr2O3, TiCl3, VCl3 ) and better oxidation nation covalent and acidic (V2O5, MnO3, Mn2O7, VCl4, and VOCl3 ).
- Lower oxidation countries may also stabilize by returning bonding in complexes. Ni(CO)four, Fe(CO)5, [Ag(CN)2]–, [Ag(NH3)2]+
Lower oxidation states in those metals are stabilized with ligands like CO, which are pi-electron donors. In contrast, the higher oxidation states are stabilized with electronegative factors like Fluorine(F) and Oxygen(O). Hence the high oxidation compounds of these metals are mainly fluorides and oxides.
Eight. Relative stabilities of the oxidation states depend on many factors, like, the stability of the resulting orbital, IE, electronegativity, enthalpy of atomization, enthalpy of hydration, and so forth.
Ti4+ (3d0) is stronger than Ti3+(3d1). Mn2+ (3d5) is extra solid than Mn3+(3d4).
Ionization energies contribute to the relative balance of transition metal compounds (ions). For instance, Ni2+ compounds are thermodynamically more stable than Pt2+, whereas Pt4+ compounds are more stable than Ni4+.
Thus, the ionization of Ni to Ni2+ requires lesser power (2490 kJ mol−1) compared to the electricity required for manufacturing Pt2+ (2660 kJ mol−1). Therefore, Ni2+ compounds are thermodynamically more strong than Pt2+ compounds.
On the other hand, the formation of Pt4+ requires lesser strength (9360 kJ/mol1) in comparison to that required for the formation of Ni4+ (11290 kJ/mol). Therefore, Pt4+ compounds are stronger than Ni4+ compounds. This is supported by the reality that [PtCl6]2+ complexion is thought, at the same time, as the corresponding ion for nickel isn’t acknowledged.
Nine. In p-block, the heavier factors choose lower oxidation states due to what’s referred to as the inert pair impact. But in the case of d-block factors, the better oxidation states are greater stable for heavier participants in a set.
Electrode Potential in D Block Elements
Relative stabilities of transition steel ions in exclusive oxidation states inside the aqueous medium can be expected from the electrode ability facts. The oxidation state of a cation for which ΔH(ΔHsub + lE + ΔHhyd) or E° is greater (for less fine) will be extra stable.
E° becomes less terrible along the collection, indicating a better balance of the decreased nation.
Compared to first and second-institution metals, transition factors have low E°.
Physical Properties of D Block Elements
Density: Among the transition collection, the fashion in density could be the reverse of atomic radii, i., E. Density growth stays nearly the same and then decreases alongside the length.
Down the column density of the 4d collection is greater than 3d. Due to lanthanide contraction and larger lower atomic radii, the volume density of 5d series transition factors is double that of the 4d collection.
In the 3d collection, scandium has the bottom and copper maximum density. Osmium (d=22.57g cm-3) and Iridium (d=22.61g cm-3) of 5d collection have the very best density amongst all d block factors.
Some relative radii of d block factors are Fe ˂ Ni ˂ Cu, Fe ˂ Cu ˂ Au, Fe ˂ Hg ˂ Au.
Why do Block Elements have high Melting and Boiling Points?
Unpaired electrons and the empty or, in part, stuffed d-orbitals form covalent bonding further to the steel bonding by using s-electrons. Because of such sturdy bonding, d-block elements have high melting and boiling factors than s and p-block elements. This fashion goes until the d5 configuration and decreases as more electrons get paired within the d-orbital.
Cr, Mo, and W own the highest melting at boiling factor in their series of factors.
Manganese (Mn) and Technetium (Tc) have 1/2-filled configurations ensuing in vulnerable metallic bonding and abnormally low melting and boiling factors.
Group12, Zn, Cd, and Hg don’t have any unpaired d-electrons and subsequently no covalent bonding. Their melting and boiling points may be the bottom of their collection.
Mercury – the liquid metal: Mercury is the simplest steel in its liquid nation at room temperature. 6s valence electrons of Mercury are greater closely pulled by the nucleus (lanthanide contraction) such that outer s-electrons are much less concerned with metal bonding.
What are Transition Elements Considered Noble Metals?
In the 3 transition series
The ionization energies of elements increase very slowly throughout a given row.
From the left of the 3d series to the right corner, 5d transition elements, density, electronegativity, and electrical and thermal conductivities increase whilst enthalpies of hydration of the steel cations decrease in value.
This indicates that the transition metals end up regularly much less reactive and extra “noble” in person. The enormously high ionization energies, increasing electronegativity, and lowering low enthalpies of hydration make metals (Pt, Au) in the decreased right nook of the d block so unreactive that they’re frequently known as the “noble metals.”
Magnetic Properties of D Block Elements
Materials are categorized by way of their interaction with the magnetic subject:
Diamagnetic: if repelled,
Paramagnetic: if attracted and
Ferromagnetic: if it can keep the larger magnetic nature even in the absence of a magnetic subject.
Paired electrons motivate diamagnetism. Unpaired electrons bring about para-magnetism, and aligned collectively unpaired electrons produce ferromagnetism. D block elements and their ions exhibit this behaviour depending on the unpaired electrons.
Unpaired electrons make contributions to ‘orbital magnetic second’ and ‘spin magnetic moment.’ However, for 3d collection, the orbital angular second is negligible, and the approximate spin-most effective magnetic second is given through the components:
µ = √[4s (s + 1)] = √[n (n + 1)] BM
In which ‘S’ is the total spin and ‘n’ is the variety of unpaired electrons. Its unit is Bohr Magneton (BM). For higher d-series, the real magnetic moment consists of components from the orbital second, similarly to the spin moment. Chromium and molybdenum possess the maximum wide variety (6) of unpaired electrons and magnetic seconds.
Formation of Coloured Ions via D Block Elements
Compounds of d-block elements have a diffusion of colours. When a frequency of light is absorbed, the mild transmitted show off a shade complementary to the frequency absorbed. Transition detail ions can take in the frequency within the seen place to apply it ways and bring seen colour.
D-d Transition
One manner is the excitation of an electron to a higher energy level. In transition detail, ions, d-electron, and empty d-orbital shall result in coloration formation. Valence electron excitation and de-excitation. This is called the d-d transition.
D-orbitals are generally degenerate and feature equal power. The presence of ligands that could shape coordinate bonds with those ions removes the degeneracy and breaks them up into agencies, e.g., t2g d-orbitals. The power distinction (∆E) relies upon the power of the incoming ligand.
Electrons within the lower d-orbitals may be excited into the better d-orbitals with the aid of absorbing energy within the seen place (λ=four hundred-700nm) and transmit (deliver) a shade complementary to it.
For instance, [Cu(H2O)6] 2+ ions soak up pink radiation and seem complementary blue-green. Hydrated Co2+ ions absorb radiation in the blue-green area and, therefore, seem pink in the sunlight.
Cupric ions are colourless, and in the presence of water, molecules grow to be blue in coloration.
- A) Colour of the ions varies with their oxidation nation. The Cr6+, as in potassium dichromate, is yellow, while Cr3+ and Cr2+ are generally green and blue, respectively.
- B) The compound’s color also depends on the complexing or coordinating group. For example, Cu2+ indicates light blue coloration in the presence of water as a ligand, but the deep blue color inside the presence of ammonia as the ligand.
- C) Transition metal ions that have:
Filled d-orbitals having no vacant d-orbitals for the excitation of electrons are colorless. Cu+(3d10), Zn2+(3d10), Cd2+(4d10) Hg2+(5d10), and Zn, Cd, Hg are colorless.
Transition metal ions that have empty d-orbitals without d-electrons are also colourless. Sc3++(3d0), and Ti4++(3d0), ions are colorless..
L-M and M-L dπ – pπ bonding
Ligands can also donate their p electrons into the empty d orbitals of metal ions. This interplay, called ligand-metal / steel-ligand or dπ – pπ bonding, might also give shade to the compounds.
Complex Formation Tendency of D Block Elements
Complex compounds are compounds in which some impartial molecules or anions are sure to be metallic. Metals that can be a part of the d block elements form many complex compounds thanks to their small ionic length, excessive price, and relative availability of d orbitals for forming bonds.
Transition metals and their ions, With their larger nuclear price and smaller length, can attract electrons. Receive lone pairs of electrons from anions and impartial molecules into their empty d-orbitals, forming coordinate bonding.
Transition elements thus form complicated molecules with CO, NO, NH3, H2O, F–, Cl–, and CN–. Examples of transition metal complexes are, [Co(NH3) 6] 3+ [Cu(NH3)4] 2+, Y(H2O) 6]2+, [Fe(CN)6]four−, [FeF6] three−, [Ni(CO)4],
Catalytic Activity of Elements
Catalysts are crucial for the industrial bulk manufacturing of many chemical compounds. Many d-block elements, as metals are in their ionic form, are used as a catalyst for many chemical and organic reactions.
Iron in Haber’s process of making ammonia, vanadium pentoxide in the manufacture of sulphuric acid, titanium chloride as Zigler Natta catalyst in polymerization, and Palladium chloride within the conversion of ethylene to acetaldehyde are some very critical industrial catalytic strategies regarding d block metals.
Most transition factors act as top catalysts due to,
- The presence of vacant d-orbitals.
- The tendency to exhibit variable oxidation states.
- The tendency to form response intermediates with reactants.
- The presence of defects in their crystal lattices.
- They take the response through a course of low-activation electricity by:
- Providing a big surface place for absorption and allowing sufficient time to react,
- May engage with the reactants through their empty orbitals.
- May actively interact by means of redox reaction through their more than one oxidation state.
Alloy Formation in D Block Elements
Atomic radii of the transition elements in any collection are not a great deal exceptional from each other. As a result, they can very effortlessly update every difference inside the lattice and form stable solutions over a considerable composition variety. Atoms inside 15% of the difference in radii can form alloys.
Such stable solutions are referred to as alloys. Alloys are homogeneous solid solutions of two metals or steel with a non-metal. The alloys of transition metals are tough, and high metals are high melting in comparison to the host metallic.
Various steel sheets are iron alloys with metals, chromium, vanadium, molybdenum, tungsten, manganese, etc. Some important alloys are:
Bronze – Cu(75-ninety%) + Sn (10-25%); Chromium metal – Cr(2-4% of Fe) Stainless metal- Cr(12-14% and Ni(2-4%) of Fe; Solder- Pb +Sn
Interstitial Compounds of D Block Elements
Transition metal has a void in its crystal lattice shape. Small non-steel atoms and molecules like hydrogen, boron, carbon, and many others may be trapped in the void during crystal structure formation. These are called interstitial compounds. They are neither ionic nor covalent and non-stoichiometric, as in TiH1.7 and VH0.Fifty-six.
Interstitial compounds have the following properties:
- Their melting factors are very excessive.
- They are extremely difficult.
- They have similar conductivity houses when compared to other metals
- They are unreactive and tend to be chemically inert.
Examples of interstitial compounds formed with transition metals are TiC, Mn4N, Fe3H, and TiH2.
Non-Stoichiometric Compounds
Transition metal compounds of different oxidation states can also occasionally be given together. They may be formed by way of strong shape defects or by means of commonplace situations. But, this combination behaves like an unmarried compound.
Get d and f block elements class 12 ncert solutions
Importance of D Block Elements topic in JEE & Board Exams
This is one of the most significant topics for students preparing for the JEE mains tests. Candidates must understand this chapter well to perform well on their tests. This topic is also important for CBSE Board Examination.
FAQs:
What are d-block elements 12?
D-block factors are discovered within the periodic table in companies 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12; d-block elements are also known as transition metals. Since they display transitional behaviour among s-block and p-block elements, those d-block elements are taken into consideration as transition elements.
How many d-block elements are there in the periodic table?
Step by step answer: According to our question, the correct answer is 40 as the number of elements present in the d-block of the periodic table is 40. D-block elements are also known as transition metals since they display momentary conduct between s-square and p-block components.
Q. Why are they called d-block elements?
Ans: They’re referred to as “D-block elements” because they have valence electrons in single or extra d-orbitals.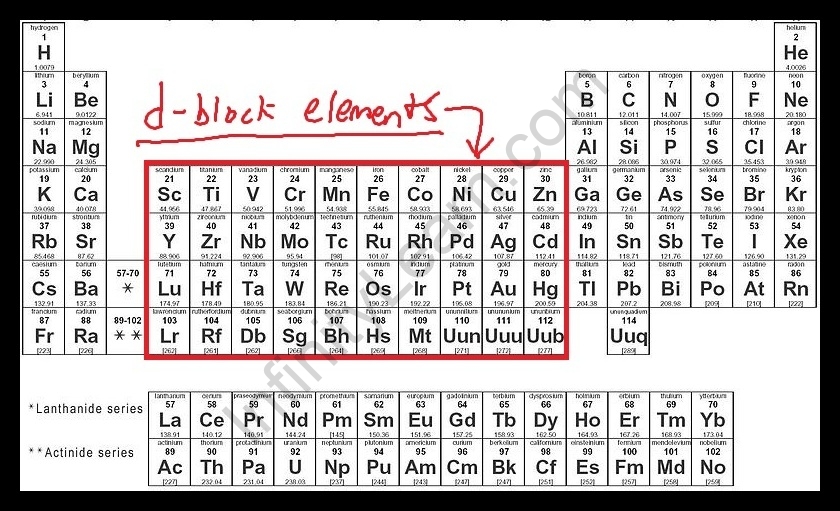
Infinity Learn App
Now you can find answers to all your subject queries & prepare for your Exams on our Learning App.









