Table of Contents
Introduction:
In this expression, effective surface energy is the specific work of destruction. The effective surface energy, together with the real surface energy s, includes the plastic deformation work per unit area of the crack surface, that is, the energy of lattice distortion and damage that occurs during crack propagation.
This mathematical formula is used to measure or calculate the surface energy or tension of any liquid or water. Based on contact angle measurements and knowledge of the surface tension of the liquid, the surface energy can be calculated. For example, we can measure the tendency of a liquid to spread (wetting) or accumulate on a surface, and we can also determine the surface energy from the shape of the droplet.
Under these conditions, and only due to the work of the surface energies of the system, which a drop of liquid spontaneously diffuses over the surface.
At equilibrium, the work done by the drop – the energy released – will lead to an energy change on the surfaces of the system. In the present study, the release of gravitational energy leads to the contour of the drop recedes to a solid flat surface and undergoing hysteresis.
In another type of Rehbinder effect, the same adsorbent-active medium, by lowering the surface energy, also promotes the development of new surfaces, which always occurs when solids are deformed. At the same time, numerous experiments have shown that the value of1 itself is very sensitive to changes in the surface energy of a solid and decreases sharply when the solid comes into contact with a strongly adsorbing medium. Another feature of the influence of the structure of a solid body on the intensity of the effects caused by adsorption is related to the excess free energy of defects.
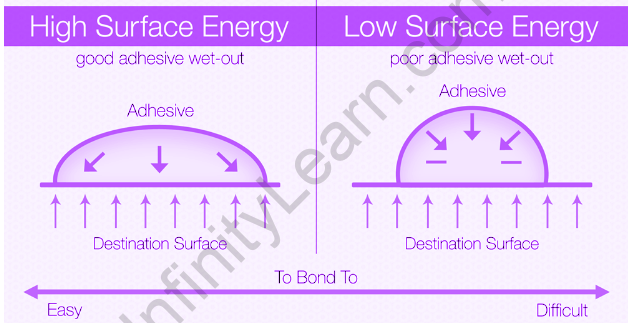
In this case, plasticity arises not due to a decrease in the resistance to plastic flow, as in adsorption plasticization, but due to an increase in the resistance of crystals due to the dissolution of the surface layer containing structural defects. . A liquid with a low surface tension will tend to spread and stick because the attractive forces of the molecules on the surface of the paper or plastic draw the molecules to the surface of the ink. As a result, molecules on the surface tend to exit and enter most of the liquid or liquid under tension and try to get the minimum area. In the liquid phase, the surface is under some tension and tends to shrink as little as possible to contain the minimum number of molecules on the outside.
“Surface tension is usually used in relation to the forces of attraction between the molecules of a liquid, while surface energy is usually used in relation to the forces of attraction between the molecules of a solid.”
Surface tension and surface energy are important because they are measurable numbers that tell us how strong the attraction between molecules is. Surface tension is literally the force required to overcome these attractions and separate the molecules present on the surface of the liquid.
In chemistry or physics, surface tension simply defines the amount of energy that increases a unit area of water or any other liquid (wetting or non-wetting). For this reason, “surface tension” and “surface free energy” are synonymous with the understanding that both are actually Helmholtz specific surface energy.
This important information about how liquids react with solid surfaces is one reason why surface tension and surface energy are so important in the manufacturing process. Manufacturers who use liquid products such as paints and adhesives to assemble and clean products ranging from microchips to military aircraft how these liquids will consistently adhere to the surfaces of their products. This method cannot be used to measure the surface energy of solids because stretching a solid film induces elastic energy in the mass and also increases the surface energy.
In this case, the amount of work g dA (where g is the surface energy density of the fluid) is required to increase the surface area of the fluid mass by dA.
To measure and interpret the surface tension or energy of fluids, we use thin films containing rectangular frames and movable membranes. Surface tension, or surface energy, is the most important property of liquids that form on surfaces and is manifested when a liquid comes into contact with its vapour or when a liquid is drawn out of an elastic membrane. It is defined as the force along the surface of the liquid at right angles to a straight line of any unit length of the liquid.
The surface energy equation is based on thermodynamic principles and is used to describe the change in vapour pressure caused by a liquid with a curved surface.
This amount is not equal to the free surface energy except under certain conditions. The term “surface tension” has been used to describe the shrinkage properties of surface films, ie their tendency to minimize surface area. There is “excess energy” due to the incomplete and unrealized connection of the two surfaces.
The second component of the work that the drop must do, the energy required to create receding wetting when it is deformed from state G to state k, can be calculated by integrating all the energy differences necessary to shift the level curve on the surface towards the radial direction.
In solid-state physics, surfaces must be inherently less energetically favourable than a mass of material (molecules on a surface have more energy than molecules in a mass of material), otherwise, there would be a buoyant force to create the surfaces. Removing most of the material (see sublimation).
FAQ’s
QUESTION: What is the surface energy formula?
ANS: E = [M1 L2 T–2] × [M0 L2 T0]–1 = [M1 L0 T–2]
What is the surface energy of a material?
A term used to describe the surface of a given substrate.
What is the surface energy of a particle?
The difference in the energy between a particle and the same number of atoms in an infinitely extended solid.






