Table of Contents
Introduction
According to JEE syllabus, unfastened radical reactions cowl an extensive range of reactions in natural chemistry. Having great information on the mechanism of loose radical reactions enables you to understand a majority of reactions in herbal chemistry.
Some FAQs associated with loose radical reactions are given below:
Q. What is a Free Radical Reaction?
Ans: Free radical reactions in organic chemistry are defined as the reactions regarding free radicals acquired from homolysis or homolytic cleavage. In such reactions, each bonding partner gets an identical range of electrons. A favoured free radical response related to hemolysis.
Q. What is the overall mechanism without fee radical reactions?
Ans: JEE syllabus includes a big range of free radical reactions. One of these reactions is the anti-Markovnikov addition.
Q. Mechanism of anti-Markovnikov addition: Reaction proceeds in several steps concerning:
Ans: Generation of free radical through homolytic cleavage.
- Attack of generated free radical on the hydrogen halide to generate halide radical thru homolysis.
- Assault of created halide extremist on alkene atom to produce alkyl revolutionary through homolysis.
- Attack of the generated alkyl radical on hydrogen halide to form alkyl halide thru homolytic cleavage of hydrogen halide bond
Q. What is a radical response example?
Ans: Radical reactions are very often initiated through mild and are not depending on the polarity of the reaction medium. Halogenation of alkanes is a superb example of unfastened radical response, esp. The chlorination of methane where chloroform(trichloromethane) and tetrachloromethane are fashioned.
Q. Which is a loose radical substitution response?
Ans: Free radicals are atoms or groups of atoms that have a single unpaired electron. An unfastened radical substitution reaction is one involving these radicals. Free radicals are fashioned if a bond splits frivolously – each atom gets one of the two electrons. The call given to this is homolytic fission.
Q. What have unfastened radicals in organic chemistry?
Ans: A loose radical can be described as an atom or molecule containing one or extra unpaired electrons in valency shell or outer orbit and is capable of unbiased lifestyles. The abnormal variety of electron(s) of an unfastened radical makes it unstable, quick-lived, and fantastically reactive.
Q. What is the distinction between radical and free radical?
Ans: A radical (frequently, but unnecessarily known as a free radical) is an atom or institution of atoms that have one or greater unpaired electrons. Radicals could have an advantageous, negative, or neutral rate.
Q. What is free radical provide an example?
Ans: A tremendous example of a free radical is the hydroxyl radical (HO•), a molecule this is one hydrogen atom quickly of a water molecule and for this reason has one bond “dangling” from the oxygen.
Q. Is chlorine a free radical?
Ans: A chlorine atom has an unpaired electron and acts as a loose radical.
Q. Is oxidation a radical substitution response?
Ans: Radical substitution reactions:
Many oxidation and reduction reactions in organic chemistry have unfastened radical intermediates, for instance, the oxidation of aldehydes to carboxylic acids with chromic acid. Coupling responses can likewise be viewed as revolutionary replacements.
Q. Why are loose radicals trouble?
Ans: Free radical’s harm contributes to the aetiology of many persistent fitness problems which include cardiovascular and inflammatory sickness, cataracts, and cancer. Antioxidants prevent free radical precipitated tissue damage by preventing the formation of radicals, scavenging them, or utilizing promoting their decomposition.
Q. How do you become aware of unfastened radicals?
Ans: ELECTRON spin resonance (ESR) spectroscopy offers a satisfactory simply to be had an approach for detecting free radicals in reacting structures. Stable unfastened radicals may be detected at concentrations as low as 10−8 M with commercially available equipment.
Q. Is ClO2 a free radical?
Ans: ClO2 is a neutral unfastened radical with C2v symmetry and its basic dipole second is 1.792 Debye. 1.1. In its floor country, even though the unpaired electron is shared amongst all three atoms of ClO2, most of the people of the electron density are living totally on either oxygen atom.
Q. Why free radical is impartial?
Ans: Unpaired electron method is an electron that isn’t always paired to some other electron of opposite spin. Atoms have unpaired electrons if they have an unusual number of electrons. It may be electrically impartial due to the fact there are as many electrons as protons, so the net rate is 0.
Q. What are the advantages of unfastened radicals?
Ans: The frame can use loose radicals for correct. This consists of killing pathogens and regulating cell growth. The immune system, for instance, takes gain of unfastened radicals’ mobile-adverse traits and uses them to ruin pathogens. Pathogens are disorder-causing organisms including microorganisms and viruses.
Q. Is singlet oxygen an unfastened radical?
Ans: Singlet oxygen is not a loose radical but it can be formed for the duration of a few loose radical reactions and may cause the formation of loose radicals.
Q. Is Lithium an unfastened radical?
Ans: You can see that lithium, which has the chemical image Li, has one valence electron this is on my own, or unpaired. This makes lithium an unfastened radical because its electron would not have an associate.
Q. What is the distinction between atom and free radical?
Ans: Atoms that are solid and exist as atoms (not remoted in a vacuum) are not radicals. They are noble gas atoms. An atom or a molecule with an unpaired electron is called a loose radical. It is exceedingly reactive and is in all likelihood to form a chemical bond with the primary issue it meets.
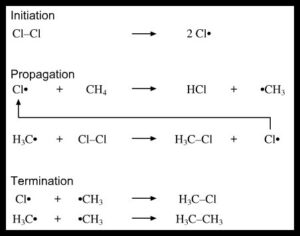
Q. What are the three steps involved in the loose radical mechanism?
Ans: Radical chain reactions have 3 awesome levels: initiation, propagation, and termination.
Q. Is loose radical substitution similar to nucleophilic substitution?
Ans: Free radicals are molecules that have an unpaired (lone) electron. This makes them very unstable, and they unexpectedly combine with different species which can be looking to benefit a valence electron. Nucleophiles can donate a pair of electrons to an electrophile thereby forming a chemical bond.
Q. What is the least solid radical?
Ans: Methyl radicals:
Specifically, tertiary radical is maximum stability and the primary and methyl radicals are least strong, which observe the same trend as the stableness of carbocations.




