Table of Contents
What is amine and give a model?
Amines are natural subordinates of smelling salts, in which one, two, or every one of the three of the hydrogens of alkali are supplanted by natural gatherings. Model: C2H5NH2.
What are instances of amines?
Amino acids, biogenic amines, trimethylamine, and aniline are central amines; see Category: Amines for an overview of amines. Alkali inorganic subordinates are otherwise called amines, for instance, monochloramine (NClH2).
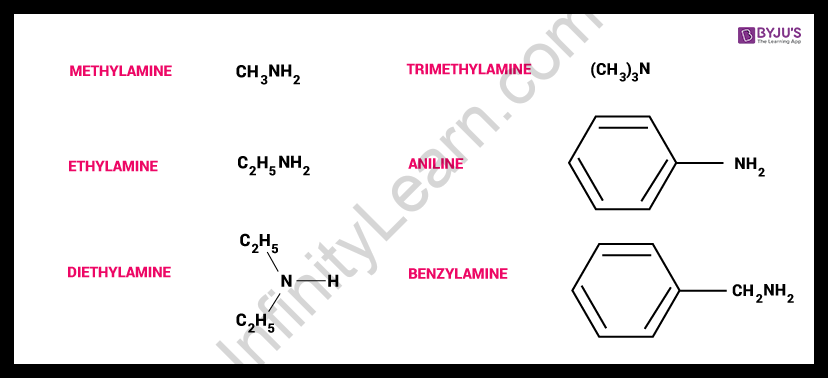
What is the amines equation?
Amine particles have the overall equation of R3-xx where R is a hydrocarbon gathering, and x is a whole number with 0 < x < 3. Put another way, amines are subsidiaries of alkali, NH3, in which at least one hydrogen particle has been supplanted by hydrocarbon gatherings. Explicit instances of amines are displayed in the following area.
What does amine do in the body?
Amines take an interest in significant metabolic and physiological capacities in living creatures. Polyamines are fundamental for cell multiplication, development, reestablishment, and digestion. They are engaged with essentially every progression of DNA, RNA, and protein union, and direct the porousness and security of cell films.
Where are amines found in nature?
Aliphatic amines happen in nature, essentially as a result of the rot of protein material, yet they are additionally present in living tissue (e.g., receptor, a cyclic aliphatic amine). The methylamines happen in limited quantities in certain plants.
What are amides utilized for?
Amides might be utilized to frame strong primary materials (e.g., nylon, Kevlar). Dimethylformamide is a significant natural dissolvable. Plants produce amides for an assortment of capacities. Amides are found in many medications.
How would you make amines?
Amines are ready from nitro compounds by ignoring hydrogen gas nitro compounds within the sight of finely separated nickel, palladium, or platinum. this reaction incorporates the reduction of nitro combinations to amines.
What is the useful gathering of amines?
Amino acids are natural particles that contain an amine practical gathering (- NH2), a carboxylic corrosive utilitarian gathering (- COOH), and a side chain (that is explicit to every individual amino corrosive).
What are amines in the cerebrum?
There are five laid out biogenic amine synapses: the three catecholamines-dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline)- and receptor and serotonin
Does espresso contain amines?
They are likewise present in beverages like tea and espresso, juices, brew, and wines. Salicylates are found in certain flavourings, fragrances, scented toiletries, and certain drugs (for example ibuprofen). Amines structure when proteins are separated and during specific cycles like maturation.
Is amine a corrosive or base?
Amine is fundamental and effectively responds with the hydrogen of acids which are electron-poor as seen underneath. Amines are one of the main impartial utilitarian gatherings which are viewed as a premise that is an outcome of the presence of the solitary pair electrons on the nitrogen.
Are amines and amides the equivalent?
Compounds containing a nitrogen particle fortified in a hydrocarbon structure are named amines. Intensifies that have a nitrogen iota clung aside of a carbonyl gathering are named amides.
Q. What is the distinction between ether and ketone?