Table of Contents
The laws of thermodynamics govern the interactions of heat with mechanical, electrical, and other types of energy or work. The laws are only valid when applied to systems in thermal equilibrium, not when applied to systems in rapid change or with complicated states of transition. A reversible system is one that is nearly always in equilibrium. The first law of thermodynamics states that heat is a type of energy, and thus thermodynamic processes are subject to the principle of energy conservation. It can, however, be moved from one location to another and converted into and out of other forms of energy. Thermodynamics is a field of physics that investigates how heat interacts with other forms of energy. The first law of thermodynamics is an adaptation of the law of conservation of energy for thermodynamic processes, distinguishing three types of energy transfer, as heat, thermodynamic work, and energy associated with matter transfer, and relating them to an internal energy function of a body. The law of conservation of energy asserts that the total energy of an isolated system (where energy and matter cannot be transferred beyond the system border) is constant; energy is capable of being transformed from one state to another, but it cannot be created or destroyed. The first law for a thermodynamic process that does not involve matter transfer is frequently stated as
ΔU = Q – W
Overview
The first law states that if heat is recognised as a type of energy, the total energy of a system plus its surroundings is conserved; in other words, the total energy of the universe remains constant. Consider the flow of energy across the boundary that separates a system from its surroundings when applying the first law. Consider the classic case of a gas contained within a cylinder with a movable piston. The cylinder walls serve as a barrier between the gas inside and the outside world, and the movable piston serves as a mechanism for the gas to do work by expanding against the (assumed frictionless) force holding the piston in place. If the gas does work W as it expands and/or absorbs heat Q from its surroundings through the cylinder’s walls, there is a net flow of energy W- Q across the boundary to the surroundings.
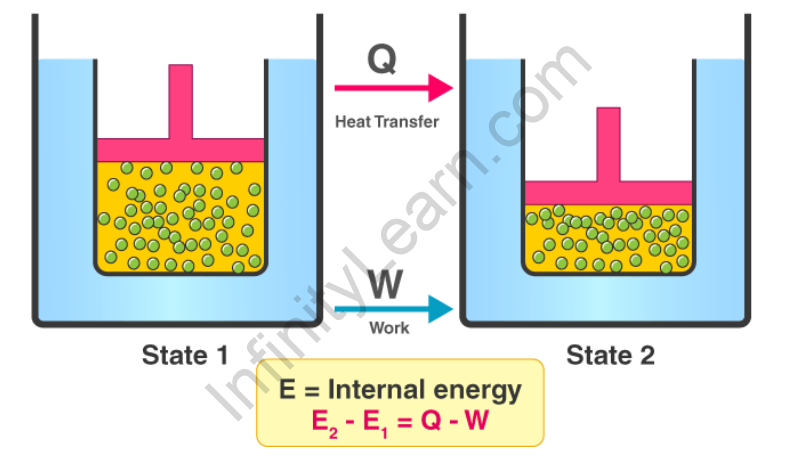
Law of Thermodynamics Equation
The first law of thermodynamics is denoted by the equation;
ΔU = Q + W
Where,
ΔU= change in the system’s internal energy.
The algebraic total of heat transmission between the system and its surroundings is denoted by the letter q.
W denotes the system’s work interaction with its surroundings.
In an isolated system, energy (E) is always constant. Internal Energy is a point function and a system attribute. Internal energy is a broad (mass-dependent) property, whereas specific energy is a narrow (mass-independent) property (independent of mass). The internal energy of an ideal gas is only a function of temperature.
Limitations of First Law of Thermodynamics
- The first law of thermodynamics has a limitation in that it says nothing about the direction of heat flow.
- It makes no distinction between spontaneous and non-spontaneous processes.
- It is not possible to reverse the process. In practice, heat does not completely convert to work. If it had been possible to convert all of the heat into work, we could drive ships across the ocean by extracting heat from the water.
The first law of Thermodynamics for a Closed System
The product of applied pressure and volume change induced by applied pressure is the work done in a closed system:
w = − P ΔV
Where P denotes the constant external pressure on the system and V denotes the volume change of the system. This is known as “pressure-volume” work.
The internal energy of a system falls or rises in response to work interaction that occurs across its boundaries. When work is done on the system, internal energy rises, and when work is done by the system, it falls. Any heat interaction that occurs in the system with its surroundings changes the system’s internal energy. However, because energy is constant (according to the first law of thermodynamics), the total change in internal energy is always zero.
If the system loses energy, the environment absorbs it. When a system absorbs energy, it implies that the energy was released by the environment:
ΔUsystem = −ΔUsurroundings
Where ΔUsystem denotes the change in total internal energy of the system and ΔUsurroundings denotes the change in total energy of the surroundings.
FAQs
What exactly are the first and second thermodynamic laws?
The first law of thermodynamics, also known as the Law of Conservation of Energy, states that energy can only be transformed or transferred, not created or destroyed. According to the second law of thermodynamics, the entropy of an isolated system always increases over time.
What is the definition of work in the first law of thermodynamics?
The first law of thermodynamics is expressed as U = Q-W, where U is the change in internal energy of a system, Q is net heat transfer (the sum of all heat transfer into and out of the system), and W is net work done (the sum of all work done on or by the system).






