Table of Contents
Introduction
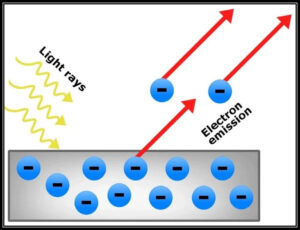
The photoelectric impact is a peculiarity wherein electrons are shot out from the outer layer of a metal when light is occurring on it. These launched out electrons are called photoelectrons. It is essential to take note that the discharge of photoelectrons and the active energy of the launched photoelectrons is subject to the recurrence of the light that is an episode on the metal’s surface. The interaction through which photoelectrons are catapulted from the outer layer of the metal because of the activity of light is usually alluded to as photoemission.
Question 1: Visible light has wavelengths in the range of 400 nm to 780 nm. Calculate the range of
the energy of the photons of visible light.
Solution:
We know, Energy of photon = hv = hc/λ
where ‘h’ represents plank’s constant
The frequency is ν
The speed of light is c and
The wavelength is λ
Now,
The energy of photon having wavelength 400 nm = [6.63×10-34x3x108]/[400×10-9]
= 4.97×10-19 J
The energy of photon having wavelength 780 nm = [6.63×10-34x3x108]/[780×10-9]
= 2.55 x 10-19 J
The range from 2.55 x 10-19 J to 4.97×10-19 J
Question 2: Calculate the momentum of a photon of light of wavelength 500 nm.
Solution:
The momentum of a photon of wavelength λ is calculated using the formula, P = h/λ
So, the momentum of a photon having a wavelength of 500 nm is:
= [6.63×10-34]/[500×10-9]
=>P = 1.32 x 10-27 kg m s-1.
Question 3: An atom absorbs a photon of wavelength 500 nm and emits another photon of
wavelength 700 nm. Find the net energy absorbed by the atom in the process.
Solution:
The energy of photon atom absorbed = hc/λ
where h is the plank’s constant and
ν represents the frequency
c is the speed of light and
λ signifies wavelength.
Thus, energy of photon atom absorbed = [6.63×10-34x3x108]/[500×10-9]
= 3.978 x 10-19 J
Then, energy of photon atom emit = [6.63×10-34x3x108]/[700×10-9]
= 2.841 x 10-19 J
Now we get,
Net absorbed energy = [Energy of photon atom absorbed] – [Energy of photon atom absorbed]
= 3.978 x 10-19 J – 2.841 x 10-19 J
= 1.137 x 10-19J.
Question 4: Calculate the number of photons emitted per second by a 10 W sodium vapour lamp.
Assume that 60% of the consumed energy is converted into light. The wavelength of sodium light =590nm.
Solution:
60% of 10 W = 6 Watts is converted into light energy.
The energy of single photon = [6.63×10-34x3x108]/[590×10-9]
= 3.371 x 10-19 J
Therefore, the number of photons required to produce 6 W energy:
n = 6/[3.371 x 10-19] = 1.7 x 1019
Question 5: When the sun is directly overhead, the surface of the earth receives 1.4 × 103 W m–2 of
sunlight. Assume that the light is monochromatic with an average wavelength of 500 nm and that no
light is absorbed in between the sun and the earth is 1.5 × 1011 m.
(a) Calculate the number of photons falling per second on each square meter of the earth’s surface directly
below the sun.
(b) How many photons are there in each cubic meter near the earth’s surface at any instant?
(c) How many photons does the sun emit per second?
Solution:
(a) The intensity at the earth surface is = 1.4 × 103 W m–2
(Also Given)
The energy of one photon = [6.63×10-34x3x108]/[500×10-9] = 3.978 x 10-19 J
Therefore,= [1.4 × 103]/[3.978 x 10-19]
= 3.519 x 1021
(b) In a unit sec all the photons which are at height, 3×108 m fall in unit m2.
The photons which fall from 1 m height will fall in (1/3) x 10-8sec.
If there are 3.519 x 1021 photons that fall in 1 sec, then, the number of photons which fall in (1/3) x 10-8 sec is (1/3) x 10-8 x 3.519 x 1021 = 1.173 x 1013, and these photons are in 1 m3 near the earth surface.
(c) From the above output, we can say that, the number of photons that fall on the surface of
sphere of the radius is equal to the total no of photons that will be produced by the sun in 1 s.
The surface area of the sphere is given as= 4π(1.5×1011)2 m square.
Thus, the total number of photons = 4π(1.5×1011)2x 3.519 x 1021
= 9.89 x 1044
Question 6: A parallel beam of monochromatic light of wavelength 663 nm is incident on a totally
reflecting plane mirror. The angle of incidence is 60° and the number of photons striking the
mirror per second is 1.0 × 1019. Calculate the force exerted by the light beam on the mirror.
Solution:
Given,
The wavelength of the monochromatic light = λ = 663 nm = 663 x 10-9 m
The angle of incidence is 60° and the number of photons striking the mirror per second is 1.0 × 1019.
As we already know,
Momentum of photon can be calculated as = p = h/λ = [6.63×10-34]/[663×10-9] =10-27
So,
Force applied on the wall = F = n[p cosθ – (-p cos θ)] = 2 np cos θ
= 2x1x1019x10-27 x ½
= 10-8 N
Question 7: A beam of white light is incident normally on a plane surface absorbing 70% of the
light and reflecting the rest. If the incident beam carries 10 W of power, find the force exerted by it on the surface.
Solution:
From the given statement, we have
Force, F = 7/10(absorbed) + 2x(3/10) (emitted) …(1)
Using the relations mentioned below,
λ = h/p …(a)
E = hc/λ …(b)
P(Power) = E/t ……(c)
F = p/t …(d)
Where p = momentum
Dividing both sides of equation (a) and (b) by t
(a)=> λ/t = h/pt or p/t = h/λt and
(b)=> E/t = hc/λt = pc/t (using above result)
Using above result in (c), we get
P = E/t = pc/t
=> P/c = p/t
(d)=> F = P/C
Now,
(1)=> F = 7/10 x P/c + 2x(3/10) x P/c
Where, P = 10 watt (given)
c = speed of light = 3×108 m/s
=>F = 4.33 x 10-8 N
Question 8: A 100 W light bulb is placed at the center of a spherical chamber of radius 20 cm. Assume that 60% of the energy supplied to the bulb is converted into light and that the surface of the chamber is perfectly absorbing. Find the pressure exerted by the light on the surface of the chamber?
Solution:
Power = 100 W
Radius R = 20 cm = 0.2 m
Power of light , say P = 60 W
We already know, F = P/c = 60/[3×108] = 2 x 10-7 N
Again, Pressure = Force/Area
p=F/A
= [2×10-7]/[4×3.14x(0.2)2] [Since, Surface area of sphere = 4πr2]
=> Pressure (p) = 4 x 10-7 Nm-2.
Question 9: A sphere of radius 1.00 cm is placed in the path of a parallel beam of light of large
aperture. The intensity of the light is 0.50 W cm–2. If the sphere completely absorbs the radiation
falling on it, find the force exerted by the light beam on the sphere.
Solution:
Intensity of light = I = 0.5 Wcm-2
Radius of the sphere is = r = 1 cm
Force F = P/c
Where P = IA;
and c = 3×108
=> F = IA/c
=> F = [0.5 x 3.14 x 12]/[3×108]
=> F = 5.2 x 10-9 N.
FAQs
What do you mean by threshold energy for the Photoelectric Effect?
For the photoelectric impact to happen, the photons that are occurring on the outer layer of the metal should convey adequate energy to beat the alluring powers that tight spot the electrons to the cores of the metals. The base measure of energy expected to eliminate an electron from the metal is known as the threshold energy (signified by the image Φ).
What are the properties of photons?
For a photon, all the quantum numbers are zero. A photon has no mass, charge and they are not reflected in an attractive and electric field. The photon moves at the speed of light in void space. During the cooperation of issues with radiation, radiation acts as it is comprised of little particles called photons.
What is the Photoelectric Effect formula?
The Photoelectric Effect formula can be signified as, hν = W + E.