Table of Contents
In 1915, Neil Bohr proposed the Bohr model of the atom. It was created as a result of a modification to Rutherford’s atomic model. Rutherford’s model introduced the nuclear model of an atom, in which a positively charged nucleus is surrounded by negatively charged electrons. The atomic structure model was modified by explaining that electrons move in fixed orbitals (shells) and not anywhere in between, and that each orbit (shell) has a fixed energy level. Rutherford essentially explained an atom’s nucleus, and Bohr modified that model to include electrons and their energy levels.
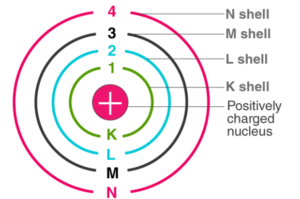
Bohr’s model consists of a small (positively charged) nucleus surrounded by negative electrons that move in orbits around the nucleus. Bohr discovered that electrons located away from the nucleus have more energy than electrons located close to the nucleus.
Bohr tweaked that view of planetary electron motion to match the regular patterns (spectral series) of light emitted by real hydrogen atoms. Bohr was able to account for the series of discrete wavelengths in the hydrogen emission spectrum by limiting the orbiting electrons to a series of circular orbits with discrete radii. He proposed that light emitted from hydrogen atoms only when an electron moved from an outer orbit to one closer to the nucleus. The energy lost by the electron during the abrupt transition is exactly the same as the energy of the emitted light quantum.
Question: When white radiation is passed through a sample of hydrogen gas at room temperature, only absorption lines in the Lyman series are observed.
Explain.
Solution:
White radiations are x-rays with energies ranging from 5 to 10 eV. When white radiation is passed through a sample of hydrogen gas at room temperature, only absorption lines in the Lyman series are observed. At room temperature, nearly all atoms are in their ground state. For a transition from n = 1 to n = 2, the minimum energy required for absorption is 10.2 eV. Photon radiations with an energy of around 10.2 eV are found in white radiation. As a result, they are only adequate for transmitting an electron from the n = 1 to the n = 2 level. As a result, the absorption lines are only visible in the Lyman series.
FAQs
In Bohr's model, how do electrons move?
The theory states that electrons in atoms travel in circular orbits around a central nucleus and can only orbit stably at a specific set of distances from the nucleus in certain fixed circular orbits. These orbits are associated with specific energies and are also known as energy shells or energy levels.
Bohr discovered electrons in what way?
Bohr was the first to discover that electrons move around the nucleus in different orbits and that the number of electrons in the outer orbit determines an element's properties.





