Table of Contents
Introduction
Bromine Trifluoride is generally utilized as a solid fluorinating specialist as it is a solid interhalogen compound. Both Bromine and Fluorine are incandescent light. This compound typically exists in a fluid-structure and has a seriously impactful scent. The synthetic recipe for this compound is BrF3. The compound was first found in 1906 by Paul Lebeau via completing Bromine and fluorine’s response at 20 degrees celsius.
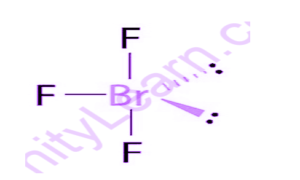
It frames a T-formed sub-atomic design and has a Bromine component as the focal molecule.
Assurance of Hybridization State
Strategy 1:
Count the accompanying sets of electrons around the focal iota:
• Count all unadulterated sigma reinforced electron sets.
• Count all solitary sets of electrons.
• Count coordinate bonds.
• Count negative charges.
Strategy 2:
To foresee hybridization the accompanying formulae might be utilized.
Number of half breed orbitals = ½ ( absolute number of the valence electron in the focal molecule + all out number of the monovalent iota – charge on cation + charge on anion )
The Hybridization of Bromine Trifluoride
To decide the hybridization of bromine trifluoride we will initially take the bromine particle which is the focal iota and check out at its electron setup. It is addressed as;
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5. Be that as it may, to frame bonds with the fluorine iota a few electrons in Bromine are moved to 4d-orbitals. Moreover, fluorine has a higher oxidative limit and in this way, it powers bromine to elevate electrons to the said level. Presently, bromine can utilize the d-orbitals for hybridization.BrF3 will comprise of seven electrons in its furthest shell. After the bond development, it will additionally have 2 solitary sets and 3 Br-F covalent bonds. As the hybridization esteem or the electron pair is equivalent to 5 it leads to sp3d mixture orbitals.
Geometry and Bond Angle of BrF3
BrF3 has three fortified and two non reinforced electrons, which gives it a three-sided pyramidal math and a T-formed sub-atomic design. Their bond points are a little compacted when we contrast it with ordinary three-sided bipyramidal design and this pressure in bond point is because of solitary sets fanning out more in space than the fortified sets. The bond point in BrF3 is somewhat lesser than 90 degree. What’s more, the purpose for the lower security point is because of the solitary pair-security pair aversion, so the precise proportion of the bond point of BrF3 is 86 degree.
Properties of Bromine trifluoride
The physical and compound properties of this substance are examined underneath:
-
Appearance:
It is a straw-shaded fluid.
-
Smell:
The substance has an impactful smell.
-
Hygroscopy:
It is a hygroscopic fluid.
-
Molar Mass:
The molar mass of Bromine trifluoride is 136.90 g/mol.
-
Monoisotopic Mass:
The monoisotopic mass of this substance is 135.913547 u (brought together nuclear mass units).
-
Thickness:
The thickness of the substance is 2.803 g/cm3.
-
Dissolving Point:
The dissolving point of Bromine trifluoride is 8.77°C.
-
Edge of boiling over:
The edge of boiling over of this substance is 125.72°C.
-
Conductivity:
Because of autoionization, the fluid is a decent conduit of power.
-
Destructiveness:
It is a destructive fluid.
-
Explicit gravity:
The particular gravity of Bromine trifluoride is 2.81 at a temperature of 68.0°F.
-
Dipole second:
The atomic dipole snapshot of this substance is 1.19 Debye.
-
Dissolvability:
Bromine trifluoride is dissolvable in sulfuric corrosive. It decays and detonates when it interacts with natural mixtures and water. Responses with hydrogen-containing mixtures can cause brutal responses. Numerous ionic fluorides effectively disintegrate in Bromine trifluoride and structure solvobases.
Is BrF3 polar?
The obligations of Br-F are considered polar in view of a somewhat high contrast in electronegativity upsides of fluorine and bromine iotas in the compound. The unshared sets or the solitary sets are situated in the plane of the triangle, causing a lopsided appropriation of negative charge around the focal bromine iota and, thusly, makes the compound profoundly polar. Subsequently one can say that Bromine trifluoride is polar.
Storage of Bromine trifluoride
The substance ought to be put away and dealt with as per the current norms and guidelines of NFPA 430 Code for the capacity of Liquid and Solid Oxidizing Materials. The compound compartments ought to be safeguarded from any sort of actual harm. They ought to likewise be avoided any incongruent substances.
Bromine trifluoride Uses
Bromine trifluoride is a solid ionizing inorganic dissolvable and fluorinating specialist. It is likewise utilized for assembling uranium hexafluoride (UF6) while handling and going back over atomic fuel.
Bromine trifluoride Health Hazards
A person presented to Bromine trifluoride can experience the ill effects of the accompanying medical issues:
- Skin contact: Skin contact with this compound can prompt consuming and bothering sensations.
- Eye to eye connection: If the eyes interact with the compound fumes or the actual substance, it can prompt extreme consuming of the eyes, ulcers and even visual deficiency.
- Ingestion: Ingestion prompts extreme consuming of the mucous layers.
- Inward breath: Inhalation can prompt serious bothering of the upper respiratory framework.
FAQs
What is the shape of BrF3?
BrF3 atomic math is supposed to be T-molded or Trigonal Bipyramidal with a bond point of 86.2o which is somewhat more modest than the typical 90°. This point framed because of the aversion produced by the electron sets which is more prominent than that of the Br-F bonds.
What is BrF3 utilized for?
Bromine trifluoride is a solid ionizing inorganic dissolvable and fluorinating specialist. It is additionally utilized for assembling uranium hexafluoride (UF6) while handling and going back over atomic fuel.
What number of electron areas does BrF3?
The BrF3 has seven electrons in the peripheral shell for hybridization. Br and F will shape bonds and will have two solitary sets and three covalent bonds.
What number of 90-degree points are available in BrF3?
The 2 solitary sets are near one another over the Br and the 2 F particles are 180° separated evenly and one F iota is in vertical hub. So the quantity of F-Br-F bond with 90° will be 2.
What is the oxidation province of F in BrF3?
Bromine trifluoride is an impartial compound with the amount of oxidation number equivalent to nothing. By the guidelines of allocating oxidation numbers the oxidation number of a gathering 17 component in a double compound is - 1. Fluorine has a place with bunch of 17 components. In this way, the oxidation number of fluorine is - 1.








