Table of Contents
Hybridization Of Phosphine
Introduction
Orbital Hybridization is one of the main points in current Physical Chemistry. Orbital Hybridization for the most part alluded to as Hybridization in science is an idea that portrays the joining of the blending of nuclear orbitals to shape new orbitals. These new orbitals are different in shape and energies when contrasted with the orbitals that are consolidated to frame these orbitals. The new orbitals are along these lines called half breed orbitals. The mixture orbitals are reasonable for the matching of electrons in the valence bond hypothesis to shape substance bonds.
Cause for Hybridization
Hybridization is a peculiarity that happens when a molecule makes a bond with the other particle with the assistance of the electrons that are from both ‘s’ and ‘p’ orbitals. This sort of synthetic holding makes an unevenness in the energy levels of the two electrons. To balance out this variety in energy levels of the electrons from two unique orbitals, the orbitals that hold the electrons engaged with bond development consolidate to frame a crossover orbital
Construction of PH3
The normal name of PH3 is phosphine. Phosphine has no trademark tone. Notwithstanding, it is an inflammable and poisonous gas. It is recognized as a pnictogen hydride.
- However, phosphine in its most perfect structure has no trademark scent, the specialized grade tests of phosphine smell with an undesirable scent of garlic or spoiled fish.
- It is tracked down that the presence of subbed phosphine and diphosphine actuates this unsavoury scent. The atomic equation of Phosphine is PH3.
- This demonstrates that the construction of phosphine ought to incorporate one phosphorus and three hydrogen molecules bound together.
- The bond point in PH3 is 93o C. The math of its design portrays phosphine as a three-sided pyramidal particle. Since phosphine is vaporous at room temperature, it’s bubbling and dissolving focuses are relatively low.
- The dissolving point of phosphine is – 132.8o C and its edge of boiling over are – 87.7o C. Phosphine has a molar mass of 33.99758 g/mol and is profoundly dissolvable in water.
Hybridization in Phosphine
It is very astonishing to portray hybridization in Phosphine. This is on the grounds that it’s obviously true that Phosphine has a distinct orbital design and electron appropriation. At the end of the day, we can essentially say that the course of hybridization isn’t legitimate on account of a phosphine particle. The resulting areas of this page will give a short outline of the shortfall of hybridization in Phosphine particles.
The definite investigation of the construction and development of the phosphine particle gives an agreement that the electrons in unadulterated ‘p’ orbital will participate in the arrangement of synthetic securities. This goes about as an opposition for the orbitals to get hybridized.
- The solitary pair of electrons are mostly in the ‘s’ orbital and ‘s’ orbital is the solitary pair orbital. Phosphorus will accordingly have three bond sets and one solitary pair of electrons.
- A definite clarification of the shortfall of PH3 hybridization is given by Drago’s standard. PH3 is viewed as a Drago particle.
- For the unadulterated ‘p’ orbitals that hold the electrons associated with security arrangement, the security point is almost 900. The Lewis dab construction of Phosphine empowers us to comprehend that the Phosphine is three-sided and pyramidal.
Drago’s Rule and Hybridization of Phosphine
Drago’s standard expresses that there is no requirement for thinking about the hybridization of a component in the accompanying cases:
Case 1: At least one solitary pair of electrons is available on the focal iota of the atom.
Case 2: Any of the components from bunches 13, 14, 15, 16 or from the third to seventh period frames the focal iota.
Case 3: The focal molecule has an electronegativity not exactly or equivalent to 2.5.
Case 4: Sigma bonds are missing and 4 solitary sets are there.
- Allow us to think about the phosphine particle. In this atom, the focal component is Phosphorus.
- Phosphorus is a component that has a place with the fifteenth gathering and the third time of the advanced intermittent table.
- The electronegativity of phosphorus is 2.19. Likewise, the phosphine particle has one solitary pair of electrons on the phosphorus molecule.
- Taking into account this multitude of raw numbers, the hybridization is missing in the phosphine atom as indicated by Drago’s standard.
- Nonetheless, nuclear orbitals in phosphine cross over on each other to frame compound bonds.
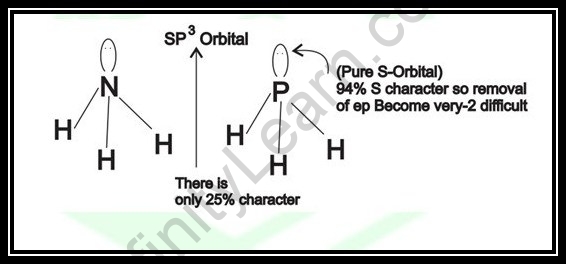
How about we have further knowledge on hybridization in Phosphine. It very well may be determined that, in the P – H bonds, just 6% of the s – character will be recorded. Taking into account that there are three P – H bonds in the phosphine particle, the s – character taking all the three P – H bonds together is 6 x 3 = 18 %.
With this computation, we can construe that the solitary pair of electrons isn’t in this orbital and it is available in the orbital which has 100 – 18 = 82% ‘s’ character. Nonetheless, it is demonstrated that none of the hybridized orbitals will have such a higher level of s-character.
This shows that the solitary pair of electrons in the PH3 atom isn’t in any of the hybridized orbitals. It is available in the unadulterated s – orbital.
Fun Facts
- Orbital hybridization doesn’t occur on account of phosphine atoms.
- The bond point PH3 is roughly equivalent to 900.
- Unadulterated ‘p’ orbital electrons are associated with the P – H security arrangement of phosphine atoms.
FAQs
Q: What are the various kinds of Hybridization?
Ans: Orbital hybridization is the most common way of blending or consolidating shaky nuclear orbitals to frame cross breed orbitals to adjust the energy level of electrons in various orbitals. Intermixing or hybridization of unadulterated nuclear orbitals is dissected before the security arrangement to present the greatest dependability of atoms. The various sorts of hybridization are:
- sp hybridization-Beryllium Chloride (BeCl2 ), Acetylene (C2 H2 )
- sp2 hybridization-Boron Trichloride (BCl3 ), Ethylene (C2 H4 )
- sp3 hybridization-Methane (CH4 ), ethane ( C2 H6 )
- sp3 d hybridization-Phosphorus Pentachloride (PCl5 )
- sp3 d2 hybridization-Sulfur Hexachloride (SF6 )
- sp3 d3 hybridization-Iodine Heptafluoride (IF7 )
How would you observe whether the hybridization is sp2 or sp3 in hydrocarbons?
Dominating the methods of deciding hybridization goes quite far in this section as well as the entire subject overall. Every one of the alkanes have focal carbon particles that are sp3 hybridized with tetrahedral math. Nonetheless, on account of alkenes and alkynes, the carbon iotas with twofold and triple bonds individually are sp2 and sp hybridized separately. The alkenes have a three-sided planar calculation. We can likewise involve a technique wherein we work out the iotas in addition to the solitary sets for any bond type and not the numerous bonds, ie., deciding the number of gatherings that are in every molecule. This is known as the Steric Number. For instance, in the event that the steric number is 4, it is sp3; in the event that the steric number is 2, it is sp. In this way C1 -SN= 3 (three molecules) so it is sp2.
Q: What are the principles for the course of Hybridization?
Ans: There are sure principles that are considered for hybridization are follows:
- The number of nuclear orbitals blended consistently approaches the number of cross breed orbitals
- The blending of the number of orbitals, in the hybridization interaction, is according to the prerequisite.
- Crossbreed bonds are more grounded than non-crossover bonds.
- Just the orbitals of the focal particle can go through hybridization.
- The half breed bonds will more often than not be farthest separated and are appropriated in space.
- The orbitals of comparable energy can be blended to frame half and half orbitals.








