Table of Contents
Hybridization Of Sf4: Sulfur tetrafluoride (SF4) is a chemical compound. It develops into a colorless corrosive gas that creates poisonous HF when it comes into touch with water or moisture. Despite its negative characteristics, this molecule is an essential reagent in producing organofluorine compounds, some of which are useful in the pharmaceutical and specialty chemical industries.
What is Sulphur Tetrafluoride Hybridization?
To figure out how sulfur tetrafluoride hybridizes, you must first figure out its Lewis structure and the number of valence electrons it contains. The SF4 molecule has 34 valence electrons in total. Each of the four fluorine atoms will have seven electrons, whereas sulfur will offer six.
The sulfur atom will make bonds with each of the fluorine atoms using eight valence electrons during the creation of SF4. In the meantime, the four fluorine atoms will have three lone pairs of electrons in their octet, with 24 valence electrons. In addition, two electrons in the sulfur atom will be maintained as lone pairs. By counting the number of electron density zones, we can now calculate sulfur’s hybridization. In the case of sulfur, bonding produces four single bonds and one lone pair. We can deduce that there are five electron density zones based on this.
Five sp3d hybrid orbitals are generated by hybridizing the five valence atomic orbitals on the central S atom. In 2P-orbitals, four hybrid orbitals overlap, with the fifth comprising a lone pair. The steric number can also be used to figure out how many hybrid orbitals an atom has. Five orbitals will be used in Sulphur, including one 3s, three 3p, and one 3d orbital.
Important Things to Keep in Mind
- On the central sulfur atom, which is coupled to four fluorine atoms, there is one lone pair.
- Five hybrid orbitals have been created.
- One 3s orbital, three 3p orbitals, and one 3d orbital are used in hybridization.
Bond Angles And Molecular Geometry Of SF4
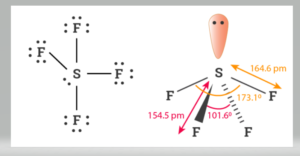
With one pair of valence electrons, the molecular geometry of SF4 is a see-saw. The molecule is polar in nature. These atoms create a trigonal bipyramidal structure. Instead of the normal 120o bond angle, the equatorial fluorine atoms have 102° bond angles. The axial fluorine atom angle is 173 degrees, rather than the standard 180 degrees.
Significance of Hybridization Of Sf4 in IIT JEE exam
It is critical to take a holistic approach to every facet of a subject’s chapter. It will not only adequately prepare you for the exam, but will also clarify your understanding of each topic. It will help you in IIT preparation and answer conceptual problems in the exam.
FAQs
In SF4, what is the S atom's hybridization?
Four fluorine atoms are linked to the lone pair on the S atom in SF4 (S has 6 valence electrons; 4 of them undergo bonding with 4 Fluorine atoms while the other 2 remains as a lone pair on S atom). All of them are in hybridized orbitals. There are five orbitals in this category. Hybridization is sp3d as a result of this.
What is the sulphur hybridization in sp3d?
Sulphur contains a total of five electron density regions, including four bonded pairs and one lone pair. As a result, there are five hybridised orbitals on the sulphur atom: one 3s, three 3p, and one 3d orbital. The arrangement of electrons surrounding the atom and hybridised orbitals causes sp3d hybridization.
How do you find sulphur tetrafluoride hybridization?
To determine how sulphur tetrafluoride hybridises, you must first determine its Lewis structure and amount of valence electrons. The SF4 molecule has 34 valence electrons in total. Each of the four fluorine atoms will have seven electrons, six of which will be supplied by sulphur.
Infinity Learn App
Now you can find answers to all your subject queries & prepare for your Exams on our Educational App – Infinity Learn.






