Table of Contents
Introduction:
The theory of the valence bond predicts the value of an atom to equal the number of unpaired electrons or slightly filled orbits, existing in their place as bonds formed by orbitals consisting of uncoiled electrons of two atoms may be different or identical.
However, the electronic configuration of the lower atoms of certain atoms indicates valency which may or may not be equal to its actual valency in their compounds. For example, a Be-atom does not have an unbroken electron (or partially orbital particle) in its base state. This setting indicates that Be cannot form any covalent bond. However, it is found that divalent in its compounds such as BeH2, BeCl2, etc. A very simple picture of atoms of half-orbital atoms cannot be used for narrative in all cellular structures. For example, consider the composition of methane (CH4) from carbon. Four C-H bonds in the case of methane are made up of a series of four carbon orbitals filled with a component consisting of four orbitals filled with hydrogen atoms.
In the case of carbon dioxide, s and p orbitals have different energies. Therefore, four carbon bonds must be of two types. Three bonds must be of the same type (s-p bond) while the fourth bond must be of the other type (s-s bond). However, experimental evidence suggests that in all four bonds in the case of CH4, the concept of Hybridization is applied. The concept of mixing is also useful in defining the visual values of bond angles.
Hybridization is the concept of a combination of atomic orbitals with the same force to give an orbital exactly the same force, the same shape, and symmetrical position in space.
Students can easily determine the hybridization of XeO2F2 by knowing the number of electrons in valence and using the basic formula for the combination given – Number of electrons = ½ [V + N-C + A].
Here V is the number of valence electrons in the middle atom (xenon).
N will be equal to the number of monovalent (fluorine) atoms combined with the central atom. C will be the cation charge and A will be the anion charge.
Example :
Molecular Name: Xenon Dioxide Difluoride
Molecular Formula: XeO2F2
Hybridization type: sp3d
Bond angle: 91 °, 105 °, and 174 °
Geometry: Trigonal Bipyramidal or See-Saw
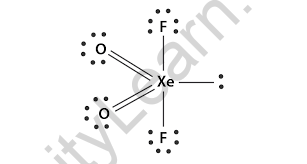
What is the Hybridization of XeO2F2?
At Xenon Dioxide Difluoride, xenon will be the central atom that will have 8 electrons in valence. The fluorine atom will be the surrounding atom and the oxygen atom will be the surrounding atom. We will take eight valence electrons from Xenon and add 2 atoms of monovalent fluorine atoms. The total amount will be divided by 2 at the end.
If we take the prices then;
Electron number = ½ [8 + 2-0 + 0]
= 5
We get 5 as the last number which also suggests an sp3d mix. For Xenon Dioxide Difluoride, there will be 5 sp3d hybrid orbitals. There are 5 pairs of electrons around the center atom where it will contain 4 bond pairs and one single pair.
Important Points to Remember
- The central atom’s Xenon hybridization of is sp3d.
- The central atom will consist of 4 bonds and 1 pair each.
- Xenon basically belongs to the respected gas family. The implication of this is that it does not have to build any bonds to complete its octet.
XeO2F2 for Molecular Geometry and Bond Angles
XeO2F2 molecular geometry is initially called bipyramidal triangular but due to the presence of a single pair in the equator, the actual shape will be observed. The disgust between the bond pair and one pair of electrons will be even greater. In this case, oxygen will be an atom in the equator and fluorine will form axial atoms. For angles, the O-Xe – O angle will be 105.7 °, the O-Xe – F will be 91.6 ° and the F – Xe – F will be 174.7 °.
Tips for Predicting the Type of hybridization of the central atom in a Molecule or ION:
Step 1: Add the valence electron number of all the atoms present in a given molecule/ion.
Step 2: In the cation case, subtract the charge number of electrons in the cation and in the case of anion, add the number of electrons equal to the charge of the anion.
Step 3: (i) If the result obtained from step 2 is less than 8, divide it by 2 and find the total quotient and the remainder.
(ii) If the result obtained in step 2 is between 9 and 56, divide it by 8 and get the first quotient. Divide the remainder (if any) by 2 and get a second quotient. Add all quotients and last resort.
Characteristics of hybridization :
- The orbitals involved in the merger should have the same strength.
- The orbitals of each atom contribute to the fusion.
- The number of compound orbitals formed is equal to the number of combined orbitals.
- Orbital hybrids are all equal in shape and strength.
- The hybrid orbital involved in the formation of the bond must have one electron in it.
- Due to the electronic reduction between hybrid orbitals, they tend to stay at a very high distance.
FAQs
What is XeOF2 hybridization?
sp3d hybridization XeOF2 includes sp3d mixing. Two pairs of single electrons are in the corners of a triangle and the molecule has a T-shape
What is the structure of XeO2F2?
XeO2F2 consists of a triangular pyramid with two π bonds and one pair of electrons with sp3d hybridization. It has 4 bond pairs and one pair of pairs which is why it shows the geometry of a triangular pyramid.
Is Xe monovalent?
In Xenon Dioxide Difluoride, xenon is a medium atom with 8 electrons in valence. The fluorine atom is the surrounding atom and the oxygen atom is the surrounding atom.
Is XeF4 sp3d2 hybridization?
By adding the number of σ bonds designed by the selected atom (in this case 'I') and the number of one pair in it, we can simply divide the mixture. In this case, 5 sigma bonds and one pair of electrons are held by IF5. That is why the sum is 6. Thus, its compound is sp3d2.
Why is XeO2F2 polar?
XeO2F2 is polar. It has 5 electrons conveyed around the Xe atom in the centre, one of which is a single pair. The upper division of the small slope means that the shape is based on a triangular bipyramid structure, but is actually bona-saw.








