Table of Contents
Introduction
The term ‘Hybridization’ refers to the formation of newly formed orbital by joining orbiting atoms. On the other hand, these newly formed orbitals affect cell geometry and binding structures.
Also, the hybridization process is an improvement in the valence bond theory. To test this information in advance, we will use three types of hydrocarbon compounds to define sp3, sp2, and sp hybridization.
As we know, in the case of XeF4 or xenon tetrafluoride, xeof₄ mixing occurs at a central atom, which is Xenon (Xe). In this case, if we look at the Xe valence shell, the total number of electrons is six in the 5p orbital and two electrons in the 5 orbital.
Let’s keep an eye on the 5th orbital; we will find that other orbitals such as the d orbital and the existing orbital do not have electrons.
There are two 5p orbital electrons present. We can say that these orbitals are in a happy state. These orbitals are transferred to complete 5d empty orbitals in the XeF4 formulation system.
This results in 4 unmixed electrons comprising 2 at 5p and 2 at 5d orbitals. So, finally, we find a real orbital used in XeF4 development, and it leads to sp2d2 synthesis.
But when we consider fluorine, there are four F atoms attached to these half-filled orbitals. The placement of fluorine atoms will be on both sides of the atom in the middle.
What is the Hybridization of Xenon Tetrafluoride?
In xenon tetrafluoride, mixing occurs with the central atom Xenon (Xe). If we look at the Xe valence shell there is a total of six electrons in the 5p orbital and two electrons in the 5s orbital. If we look at the 5th shell then there is a d orbital and an orbital where there are no electrons. In XeF4 formation, two electrons in 5p orbital particles, in a happy state, move and fill 5 d empty orbitals. As a result, there are 4 uncoiled electrons combining 2 at 5p and 2 at 5d orbitals. This results in a mix of sp3d2.
In the case of fluorine, four F atoms meet these four orbital particles. These fluorine atoms will then be placed on both sides of the middle atom.
Important Points to Remember:
- The median atom has 6 pairs of electrons, two of which are electron pairs per pair.
- The hybridization in Xenon is sp3d2 because there is a migration of two electrons p to d orbital leading to the
- Formation of a sigma bond and F.
XeOF4 Hybridization:
The s-orbital is used by the middle atom as normal and a mixture of p-orbitals and all other d-orbital together to form a hybrid orbitals.
Thus, in the case of XeOF4 formation, the orbital will be required for Xe and its three p-orbital and 2d orbital. Therefore, sp3d2 or d2sp3 will be its hybrid mode.
Brief Detail:
Title of chemical: Xenon Tetrafluoride
Chemical formula: Xef4
Type of hybridization: sp2d2
Bond angle: 90°or 180°
Structure: Square planar
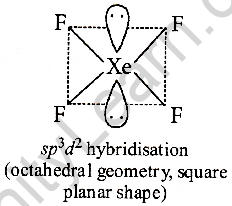
XeF4 contains two electrons per pair. Now if we follow the VSEPR theory, the erosion of electronic electronics should be minimal. With this, they will get a stable status. To achieve this, individual pairs lie on a perpendicular plane with a rectangular octahedral system (1800 degrees) from one another. Therefore, XeF4 molecular geometry is square planar.
With the help of the structure, they will get a stable condition. Xenon pairs live in a straight line with an octahedral system. This is the reason behind the geometry in the square plan of the XeF₄ molecule.
Xeof4 Structure Hybridization:
Xeof4 geometry is square pyramidal.
As we have learned before the s-orbital will use a central atom, a combination of orbitals will be mixed with its p-orbitals and their d-orbitals as they are found.
Xenon will need its orbital and its three p-orbitals in number, as well as its 2 d-orbitals to create a hybridization state such as sp3d2, or d2sp3.
Characteristics of Hybridization:
- Ions with the same energy or activation are combined.
- Hybridization occurs when orbitals of the same atom.
- Hybrid orbitals can make bonds stronger or more stable than pure atomic orbitals.
- All mixed orbitals are equal in strength and shape.
- Hybridization leads to the production of equal orbitals. This gives the orbitals a higher rating. Hybridization defines the behavior of a molecule in orbit.
FAQs
Q: What conditions are required for XeF4 Hybridization?
Ans: The following conditions are required for XeF4 Hybridization:
- All orbitals participating in the merger should have only a slight difference in the enthalpies.
- Orbital hybridization belongs to the atomic valence shell.
- Hybridization occurs between fully or partially orbital orbitals.
- It is not necessary for all orbitals of the valence shell to do hybridization on XeF4.
- Electron promotion is not a condition required for integration but the presence of at least one electron must be present.
What Is the Need for Hybridization?
Hybridization is required as it allows for the formation of stable and complete structures. At the end of the existence of hybrid orbitals, a sufficient number of electrons are obtained to complete the required bonds regardless of their number of valence electrons. Hybridization describes the formation of bonds in atoms, such as Carbon, at the orbital level.
What Are The Factors That Make Hybridization More Stable?
The presence of the character ‘kas’ will bring more stability. Yes, ‘sp’ has a very high ‘s ’character of about 50%; then sp2 with 33.33%, and sp3 with 25%. Binding power also plays a major role; i.e. bond strength = high stability.
Q: How Do We Calculate Hybridization?
Ans: We can calculate the atomic mix in a molecule by making a computer the total number of atoms connected to it.
Here, just count the atoms but not the bonds. Also, measure the number of single pairs attached to it. Finally, count the two numbers together. Below are the steps for calculating the simplification:
- Note the atom.
- Count all the other atoms connected to the selected atom.
- Then count the individual pairs.
- Enter both numbers.








