Table of Contents
Introduction
Concentrated hydrogen peroxide is an exceptionally responsive oxygen animal group and is utilized as a fuel in rocketry. The synthetic recipe for hydrogen peroxide is H2O2.
It is frequently alluded to as water with another oxygen molecule. It is acidic in nature and PH is around 4.5. It is a 100% degradable compound.
Hydrogen peroxide Chemical formula H2O2
- Atomic Weight/Molar Mass- 4.0147 g/mol
- Density- 1.05 g/cm3
- Bubbling Point- 150.2° C
- Liquefying Point- -0.43° C
Design of Hydrogen Peroxide
The design of hydrogen peroxide is non-planar. H2O2 has an open book structure with O – O turns. The dihedral point is 111°. The O-O bond length is 145.8 pm and the O-H bond length is 98.8 pm(which is equivalent to 9.88 × 10-13 m). The accompanying chart will obviously showcase what an open book structure implies.
There are two planes in this construction and each plane has one O-H bond pair, the point between both the planes is 90.2°.
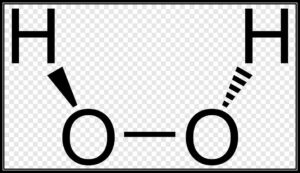
Planning of Hydrogen Peroxide
Allow us to examine the different techniques for groundwork for hydrogen peroxide.
Research centre Methods of Preparation
At the point when barium peroxide is fermented and the abundance of water is taken out by the course of vanishing under diminished pressure, we get hydrogen peroxide. The accompanying response will explain this:
BaO2.8H2O(s) + H2SO4(aq) → BaSO4(s) + H2O2(aq) + 8H2O(l)
Modern Method of Preparation
Hydrogen peroxide is ready by the electrolysis of 30% super cold H2SO4. At the point when fermented sulfate arrangement is electrolyzed at high current thickness, peroxodisulphate is acquired. Peroxodisulphate is then hydrolyzed to get hydrogen peroxide.
2HSO–4(aq) [Electrolysis] → HO3SOOSO3H(aq) [Hydrolysis] → 2HSO–4(aq)+2H+(aq)+H2O2(aq)
Response Mechanism
- Electrolyte: 30% weaken H2SO4
- Cathode: Pb wire
- Anode: Pt bar
2H2SO4 → 2H+ + 2HSO–4
At Cathode: 2H+ + 2e– → H2
At Anode:
2HSO–4 → H2S2O8 + 2e– ⇒ Peroxodi Sulphuric Acid [Marshall’s acid]
H2S2O8 + H2O → H2SO5 + H2SO4 ⇒ Peroxomono Sulphuric Acid [Caro’s acid]
H2SO5 + H2O → H2SO4 + H2O2
Business Methods Of Preparation
Auto-Oxidation Method:
Planning of Hydrogen Peroxide
Properties of Hydrogen Peroxide
Actual Properties:
- In the unadulterated state, hydrogen peroxide is practically dreary (extremely light blue) fluid.
- It liquefies at 272.4 K and has a limit of 423 K (extrapolated).
- It is miscible in water to all extents and structures hydrates.
Synthetic Properties:
Hydrogen peroxide is both acidic and fundamental mediums that go about as an oxidizing as well as a lessening specialist. The accompanying responses will give an unmistakable picture:
Oxidation Reactions of H2O2
- Oxidizes dark Pbs to white PbSO4. Response: Pbs + 4H2O2 → PbSO4 + 4H2O
- Oxidizes KI to Iodine. Response: 2KI + H2O2 → 2KOH + I2
- Oxidizes nitrites to nitrates. Response: NaNO2 + H2O2 → NaNO3 + H2O
- Oxidizes fermented Potassium ferrocyanide.
- Response: 2K4Fe(CN)6 + H2SO4 + H2O2 → 2K3Fe(CN)6 + K2SO4 + 2H2O
- Oxidizes sulphites to sulfates. Response: Na2SO3 + H2O2 → Na2SO4 + H2O
- Oxidizes CrO-24 in a corrosive medium to chromium Peroxide CrO5. Response: CrO-24 + 2H+ + 2H2O2 → CrO5 + 3H2O
Fading Reactions:
It fades by oxidation. H2O2 fades silk, fleece, cotton, hair.
Response: H2O2 → H2O + [O] Nascent oxygen
Hued substance + [O] → boring
Decrease Reactions of Hydrogen Peroxide:
- It lessens Barium Peroxide to monoxide. Response: BaO2 + H2O2 → BaO + H2O + O2
- It lessens ozone to oxygen. Response: O3 + H2O2 → H2O + 2O2
- It lessens basic potassium ferricyanide. Response: 2K3Fe(CN)6 + 2KOH + H2O2 → 2K4Fe(CN)6 + 2H2O + O2
Why Hydrogen Peroxide is Stored in Plastic Containers?
Hydrogen peroxide deteriorates when presented to daylight, this cycle is catalyzed by hints of salt metals. Thusly, H2O2 is put away in wax-lined glass or plastic compartments and kept in dull.
It ought to likewise be avoided dust particles since residue can actuate unstable decay of this compound.
Uses of H2O2
In any case, hydrogen peroxide has gained notoriety for being utilized as a purging specialist and a sanitiser. Moreover, it has been utilized as a dying specialist in different occasions and in different magnificence systems including hair, teeth and skin medicines. In labs, it is utilized as an oxidizing specialist and lessening specialist. We will take a gander at a portion of different purposes of hydrogen peroxide underneath.
- Hydrogen peroxide is utilized as a germicide.
- It is utilized in the safeguarding of wine and milk.
- Our white platelets produce hydrogen peroxide to kill microorganisms.
- It is utilized to clean messes from touchy textures and garments.
- Absorbing feet water blended in with H2O2 for 15 min consistently for seven days will assist with disposing of growth.
- Hydrogen peroxide is utilized for skin easing up and skin inflammation.
- Plant development can be supported by sprinkling a combination of hydrogen peroxide and water in equivalent sums.
- The material and paper industry use it as a fading specialist.
- It is utilized for the amalgamation of tartaric corrosive, food items and numerous drugs.
- It is utilized in the production of synthetic substances, which thusly are utilized in making top-notch cleansers.
- The main utilization of H2O2 is in ecological science where it is utilized in contamination control treatment of homegrown waste and modern effluents.
- Profoundly focused Hydrogen Peroxide is utilized as rocket fuel.
- It tends to be utilized in cleaning and disinfecting family things.
- It very well may be utilized to clean lakes and eliminate destructive green growth.
FAQs
Would it be advisable for me I use Hydrogen Peroxide to clean an injury?
Peroxide is comprised of Hydrogen and oxygen. It's a solid oxidizer and can be utilized as an attracting specialist to help contaminations. When utilized topically, this fluid foams because of the protein catalase. As the catalase comes in contact with the skin it transforms the Hydrogen Peroxide into water and oxygen gas.
What are the benefits of Hydrogen Peroxide?
Hydrogen Peroxide is a broadly utilized antimicrobial substance. It's utilized in both fluid and gas structures for additive, sanitization and disinfection tasks. Its benefits incorporate its intense and expansive diapason antimicrobial effort, rigidity being used, and wellbeing profile in contrast with other microbicides.
Q: Are there any medical advantages to drinking Hydrogen Peroxide?
Ans: 3% Hydrogen Peroxide. Additionally relating to ménage Hydrogen Peroxide, this type is for the most part used to clean or sanitize minor wounds. It’s the bone you’re probably going to find in your unique grocery store or pharmacist.
- 6 – 10% Hydrogen Peroxide. This consideration is most commonly used to fade hair.
- 35% Hydrogen Peroxide. By and large related to food-grade Hydrogen Peroxide, this assortment is for the most part planted in wellbeing food stores and elevated as a fix to vivid kind gestures and conditions.









