Table of Contents
Introduction
Homolysis (from Greek o, homoios, “equal,” and lusis, “loosening”) is a chemical bond breakdown mechanism in which each of the pieces (an atom or molecule) keeps one of the formerly connected electrons.
A brief outline
Two free radicals are produced during homolytic fission of a neutral molecule for an even number of electrons. The two electrons involved in the early bond are distributed amongst the two fragment species in this way. Bond dissociation energy is the energy involved in this situation (BDE). Bond cleavage can also be accomplished through a process known as heterolysis.
Important concepts
The homolytic fission of a molecule usually necessitates a significant amount of energy. This is why, as described below, this sort of bond fission occurs in only a few circumstances.
- When the molecule is exposed to ultraviolet radiation (electromagnetic radiation that corresponds to the ultraviolet part of the electromagnetic spectrum)
- The requisite quantity of heat to exceed the required bond dissociation energy for homolytic fission
- When carbon compounds are heated to exceptionally high temperatures in the absence of oxygen in order to enable pyrolysis, the process is known as pyrolysis.
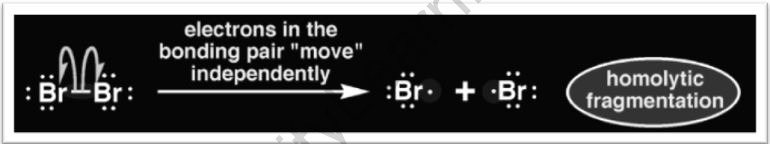
In some scenarios, homolytic fission could be produced by infusing the molecule with only a little quantity of heat. The homolytic dissociation of oxygen-oxygen bonds in peroxides is such an example. These intramolecular bonds are relatively weak, meaning that their dissociation energies are really very low. As an outcome, just a modest quantity of thermal energy is required to overcome barriers.
When comparing the bond dissociation energies for the identical types of bonds, it can be shown that the heterolytic dissociation energy is significantly larger than the homolytic dissociation energy.
Significance of homolytic in IIT JEE exam
The topic under homolytic from the chapter on some basic concepts of organic chemistry is worth around 8.33 percent of the total 120 points in the JEE test. The majority of the questions on this topic are answered using the NCERT textbook. There will certainly be a few questions concerning this topic in organic chemistry.
FAQs
The major difference between a homolytic and a heterolytic bond is that a homolytic link cleaves the covalent connection such that each fragment receives one of the shared electrons, whereas a heterolytic bond fractures the covalent bond so that one fragment receives both of the shared electrons.
A chemical bond is defined as a bond formed between two or more atoms. Chemical bonding is essential because it is what keeps the atoms together, resulting in the complex.
The strongest bond in chemistry is believed to be a covalent bond, whereas the weakest is stated to be Van der Waals forces. Is there a distinction between homolytic and heterolytic bonds?
What exactly is chemical bonding, and why is it so crucial?
Which bond is the strongest and which is the weakest?






