Table of Contents
Every natural process results in the creation of new products. Some processes known to us absorb energy, while others result in energy evolution. As a result, when processes are completed, there is always a change in enthalpy. This change in enthalpy can be attributed to the enthalpy of atomization, solution, or other factors. The enthalpy of atomization (also atomisation in British English) is the enthalpy change that occurs when all atoms in a chemical substance are completely separated (either a chemical element or a chemical compound). The symbol at ΔatH or ΔHat is commonly used to represent this. Because all bonds in the compound are broken and none are formed during atomization, enthalpies of atomization are always positive. At 298.15 K (or 25 degrees Celsius) and 100 kPa, the associated standard enthalpy is known as the Standard enthalpy of atomization, atHo/(kJ mol1).
The amount of enthalpy change that occurs when a compound’s bonds are broken and the component atoms are separated into individual atoms is referred to as the enthalpy of atomization. The symbol at ΔatH represents the enthalpy of atomization. For example, the enthalpy change of atomization of gaseous H2O is the sum of the HO–H and H–O bond dissociation enthalpies. The enthalpy of atomization of an elemental solid is the same as the enthalpy of sublimation of any elemental solid that evaporates to form a monatomic gas.
A sentence that means “turning into atoms” can be used to define atomization. Chemical reactions are carried out in the lab under continuous pressure, i.e. atmospheric pressure. Because internal energy was created specifically for volatile reactions, a thermodynamic term known as enthalpy was created to study reactions under continuous pressure. All reactions involve either the absorption or release of energy. As a result, enthalpy (H) affects temperature.
Atomization Enthalpy:
Atomization is the process of converting a solid, liquid, or solution analyte into a free gaseous atom. It is the process of converting a bulk liquid into a spray of liquid droplets in the presence of a gas or vacuum. The range of metals that can be atomized includes all metals that can be melted industrially.
The change in enthalpy when one mole of bonds is completely broken to obtain atoms in the gas phase, denoted by ΔaH0, is the enthalpy of atomization. As an example, consider the atomization of a methane molecule.
CH4 (g) → C (g) + 4H (g) ΔaH0= 1665.0 kJ mol-1
Enthalpy of atomization equals enthalpy of bond dissociation for diatomic molecules. As an example, consider the atomization of a dihydrogen molecule.
H2 (g) → 2H (g); ΔaH0= 435.0 kJ mol-1
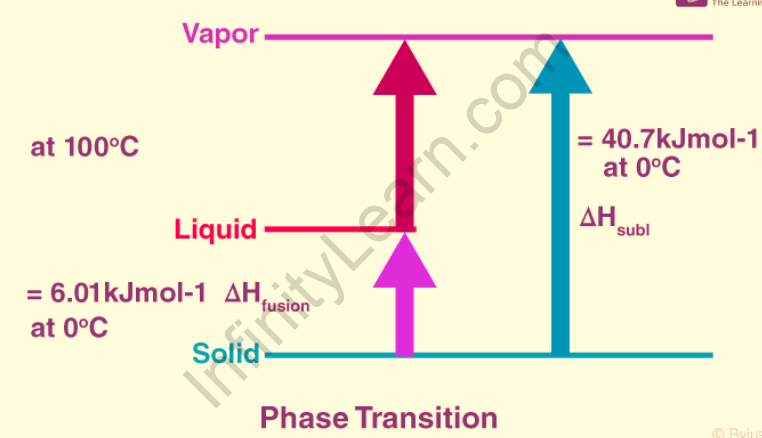
Enthalpy change during phase transition: When a substance goes through a phase transition, that is, when its phase changes from one form to another, some energy is released or absorbed. When ice melts to water, for example, energy is required for melting. The following are examples of common enthalpy changes during phase transition:
Standard vaporisation enthalpy
Standard sublimation enthalpy
Enthalpy of Atomization Is Calculated
If pressure is held constant, the enthalpy change equals the change in a system’s internal energy. As a result, the enthalpy of atomization is equal to the sum of the enthalpies of fusion and vaporisation. For example, the enthalpy of atomization of the diatomic molecule chlorine gas (Cl2) under standard conditions is simply the bond energy of Cl2. Breaking the bonds between gaseous molecules is all that is required to atomize the substance. Atomization of sodium (Na) metal at standard conditions necessitates the separation of atoms joined by metallic bonds. The enthalpy of atomization is equal to the sum of the enthalpy of fusion and the enthalpy of sodium vaporisation. The enthalpy of atomization is the same as the enthalpy of sublimation for any elemental solid.
FAQs
How is atomisation energy calculated?
When pressure is held constant, the change in enthalpy is proportional to the change in internal energy of a system. As a result, the atomization enthalpy is equal to the product of the fusion and vaporisation enthalpies.
Which of the following elements has the highest enthalpy of atomisation?
Sc and Zn are elements in the pf group's third periodic table. The amount of metallic bonding that an element has influences its atomization enthalpy. The greater an element's metallic bonding, the greater its atomization enthalpy.
What does enthalpy of atomisation mean?
Atomization enthalpy is the amount of enthalpy change that occurs when a complex's bonds dissolve and the constituent atoms are reduced to individual atoms. Ordinary atomization enthalpy is the enthalpy transition that occurs when 1 mol of material is fully dissociated through atoms under standard conditions (298.15 K, 1 bar).



