Table of Contents
Halogenation
A Halogenation response happens when at least one fluorine, chlorine, bromine, or iodine iotas supplant hydrogen molecules in the natural compound.
- The request for reactivity is fluorine > chlorine > bromine > iodine.
- Fluorine is particularly forceful and can respond viciously to natural materials.
- It additionally will in general, make the most steady of the organohalogens, and it is challenging to eliminate a fluorine molecule once added.
- Alternately, iodine is more challenging to add to a natural particle, yet once an iodoorganic structures, the iodine iota is effectively eliminated.
- In this way, the electronegativity of the halogen particle is the main thrust for halogenation responses. The responses additionally rely upon the idea of the substrate atom that is being halogenated.
Halogenations happen by a few distinct cycles relying upon the substrate: soaked hydrocarbons halogenate by means of a free extreme interaction; unsaturated organics halogenate through an expansion response; aromatics halogenate by means of electrophilic replacement.
For what reason are Halogenation Reactions Important?
Halogenation responses are significant in both mass and fine substance union and the items and intermediates produced by means of halogenation are all around addressed in drugs, polymers and plastics, refrigerants, fuel added substances, fire retardants, agro-products, and so forth
As models in drugs, fluorine or chlorine iotas added to an atom can expand the potential for its helpful movement.
- Moreover, organo-bromides and organo-iodines are exceptionally valuable as moderate mixtures, giving away to add useful gatherings to a substrate, as well as empowering the combination of more intricate constructions.
- As specific illustrations, a C-Cl or C-Br securities can be hydrolyzed to alcohols which, thus, can be oxidized to yield ketones, aldehydes and acids.
- Through disposal responses, twofold bonds can be shaped. Bromination of natural mixtures is much of the time a significant stage towards shaping a Grignard reagent, offering a manufactured pathway to construct C-C bonds.
- The alkylation of sweet-smelling rings through the Friedel-Crafts response is comprehensively pertinent and alkyl halides are basic reagents for this response.
A few significant business synthetics and items result from halogenation responses.
In an exemplary model, chloroform is fluorinated to shape chlorodifluoromethane, then changed over to fluoroethylene and polymerized to yield PTFE. Another model is the expansion halogenation of ethylene with chlorine to shape dichloroethane, which then, at that point, is polymerized to yield PVC.
Do Halogenation Reactions Need A Catalyst?
Halogenation responses might require an impetus to build the electrophilicity of the halogen.
For instance, electrophilic replacement responses of fragrant mixtures require an impetus.
Since bromine and chlorine are not electrophilic enough without help from anyone else to cause the replacement of the hydrogen, they require the presence of a Lewis corrosive.
Hence, regular impetuses for halogenation of fragrant rings are as specific illustrations, AlCl3 or AlBr3.
- Within sight of the Lewis corrosive, the holding of the halogen turns out to be more spellbound and the upgraded emphatically charged halogen is a far more grounded electrophile.
- Fluorination of aromatics doesn’t need an impetus since fluorine is a particularly solid electrophile and this response can be exceptionally enthusiastic.
- It is hard to control how much fluorine replacement happens and more than one fluorine iota may halogenate the sweet-smelling ring.
- For iodine to substitute on a fragrant ring, the metal halides are not viable impetuses, but rather an oxidizer, for example, nitric corrosive will change iodine over to HIO3 and empower the iodization of benzene.
Instances of Halogenation Reactions
Halogenations are especially valuable responses and include an expansive extent of purpose in manufactured science.
- A couple of models: Chlorination, bromination and iodization of aldehydes and ketones in the α-position are direct, however, that response with fluorine is unimaginable.
- Halogenation of the α – hydrogen in carboxylic acids with bromine or chlorine can happen by means of the Zell-Volhard-Zelinsky response, but an impetus, for example, P or PBr3 is important.
- The more straightforward it is to analyze a substrate, the simpler it is to halogenate it.
- Thus, acyl halides, anhydrides, malonic esters all go through α – halogenation without the requirement for an impetus.
- The Hoffman adjustment response utilizes bromine to change amides over to amines. Sweet-smelling rings can be brominated or chlorinated however require an impetus like Fe (really FeCl3) or AlCl3 or AlBr3.
Fluorine itself is too forceful for fluorinating aromatics, yet there are reagents, for example, ClO3F, that can be used to fluorinate certain substrates like phenols. As a rule, bromine and chlorine promptly halogenate compounds with twofold and triple bonds.
However, halogenation with X2 or HX is utilized, these atoms are regularly harmful, destructive and hard to make due. As specific illustrations, Fluorine and HF are unbelievably destructive, receptive, cause undesirable side items and overall are challenging to work with and to control response exothermicity. Hence, compounds have been fostered that can give a fluorine iota however are more steady and controllable.
For instance, diethyl-amino sulfur trifluoride (DAST) is a steady strong that changes over alcohols, aldehydes, and ketones to the relating organofluoride and is far more secure and advantageous to utilize than fluorine or sulfur tetrafluoride gases. Reagents, for example, SOCl2 and PCl5 are utilized for creating organochlorine compounds from the relating alcohols and, as on account of fluorine, simpler to utilize and control responses than with basic chlorine. As an option in contrast to bromine, N-bromo succinimide (NBS) is broadly used to brominate an alkene.
Innovation for Halogenation Reactions
Halogenations can be extremely vigorous and are delicate to dampness and air. Furthermore, response yield and selectivity are elements of the substrate, halogenating reagent, response temperature, and different factors. This mix of necessities makes the requirement for in situ examination and exact control a significant target in halogenations.
FAQs
What is the halogenation model?
Halogenation is the substitution of a hydrogen particle by a halogen iota in an atom. Incandescent light is the gathering name that is given to fluorine, chlorine, bromine and iodine. Since these components have very much like conduct, they are regularly treated collectively.
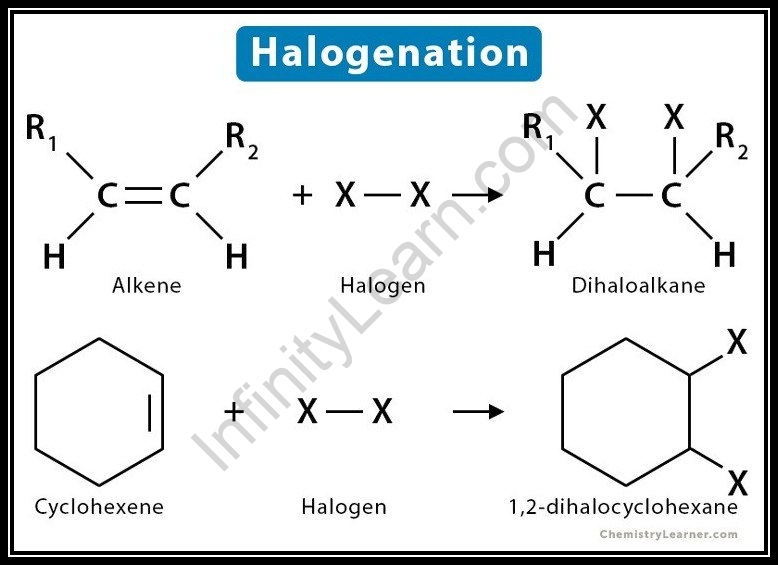
What is halogenation of alkene?
Halogenation of alkenes implies the expansion of a halogen like bromine, or chlorine at once obligation of alkenes. Halogen separates into a particle comprising of inverse charges and assaults at once attach to shape dihalide. Complete response: Hydrocarbons are the mixtures comprising of just carbon and hydrogen molecules.








