Table of Contents
Important Topic of Chemistry: Polymerization
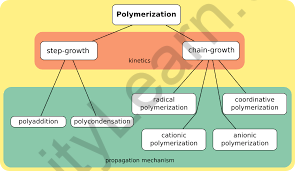
Polymerization is the chemical reaction that occurs when a high number of monomer molecules react to produce a polymer. Polymerized macromolecules can have either a linear or a branching form. They can also take the form of a complicated three-dimensional network. Polymerization processes are classified into various types, the most noteworthy of which are step-growth polymerization, chain-growth polymerization (both of which fall under the category of addition polymerization), and condensation polymerization.
A polymer is a material composed of very big molecules, which are composed of many repeating components known as monomers. Polymerization is the process through which these monomers combine to generate the macromolecules that make up polymers.
The intricacy of the polymerization reaction process might vary depending on the functional groups present in the reacting monomers. Polymers are formed from alkenes by free radical reaction in the most basic polymerization operations. Polyethene, one of the most essential economically relevant polymers, is created by this type of polymerization method (the reactant monomer used here is ethylene).
Polymerizations using just one kind of monomer are referred to as homo-polymerizations, whilst those involving more than one type of monomer are referred to as copolymerization processes. Polymerization may be defined as a chemical process that results in the creation of polymers in their most basic form.
Polymers are produced by the addition of a network of structural units or monomers. The intriguing thing about these molecules is that they are reactive and are generally bound together by covalent bonds. These monomers combine to form a lengthy chain, which results in a product with certain qualities. Polymerization refers to the entire process of polymer production. Polymers include materials such as polythene and nylon 66.
Polymerization Mechanism
Polymerization, in general, consists of three steps: initiation, propagation, and termination. In terms of the reaction mechanism, the polymerization process primarily employs two distinct approaches. The step-growth mechanism and the chain-growth mechanism are examples of these.
-
Polymerization via Step Growth
Polymers are generated by the independent reaction between the functional groups of simple monomer units in step-growth polymerization. Each step in step growth may consist of a mixture of two polymers of differing or equal length to generate a larger length molecule.
The reaction takes a long time to complete, and the molecule mass increases at a very sluggish pace. Condensation polymerization is an example of step-growth polymerization since a water molecule is generated in the process as the chain lengthens.
-
Polymerization via Condensation
The production of the polymer happens in condensation polymerization when some tiny molecules are lost as byproducts of the process when molecules are linked together. Water or hydrogen chloride may be generated as byproducts. Condensation polymers include polyamide and proteins.
Some examples of condensation polymerization are shown below.
-
Polyamides
They are synthetic fibres known as nylons. These polymers are linked together by an amide bond. Polyamide is formed via the condensation polymerization of di-amines with di-carboxylic acid, as well as amino acids and their lactams.
Nylon 66 polymer is produced under high pressure and temperature conditions by the condensation polymerization of hexamethylenediamine with adipic acid.
Nylon 6 is made by heating caprolactam in water at a high temperature. It is used in the manufacture of tire cords, textiles, and ropes.
-
Polyesters
Polyesters are generated by the polycondensation of dicarboxylic acids and diols. At 460 k, a combination of terephthalic acid and ethylene glycol is heated with zinc acetate antimony trioxide as a catalyst. The most well-known polyesters are dacron and terylene. They are also employed as glass reinforcing materials in safety helmets.
-
Polymerization via Chain Growth
The molecules of the monomers are joined together to create a big chain in chain-growth polymerization. The monomers may be of the same or a different kind. Alkenes, alkadienes, and their derivatives are often utilized. Chain lengthening occurs in this phase as a result of the production of either free radicals or ionic species.
Mechanism of Free Radicals
Many monomers, such as alkenes and dienes, and their derivatives, polymerize in the presence of free radicals. A modest quantity of benzoyl peroxide initiator is used in the polymerization of ethene to polythene by heating or exposing it to light. The peroxide-generated phenyl free radical is added to the ethene double bond, resulting in the formation of a new bigger free radical.
It’s known as a chain initiation step. This freshly produced radical will react with another ethene molecule to form still another free radical, and so on. Chain propagation refers to the recurring production of a new free radical. Finally, the polymerized product will be created at some point, which is referred to as a chain termination step.
Chemical Polymerization Reaction
When we talk about polymerization chemical reactions, we are talking to organic monomer polymerization reactions. These monomers are in a solution, which also contains particles that will be coated with the polymer that has been synthesized and placed on the particle surface. As a result, a coating layer is formed. Either monomer adsorption polymerization or emulsion polymerization is used in the process.
Polymerization Degree
The frequency of repeating units in a polymer can be used to define the degree of polymerization. For example, if a polymer P is composed of 4 monomers (M), its degree of polymerization will be 4. This is the primary property of a polymer that also specifies its physical qualities.
FAQs
How reversible is the process of polymerization?
Under some conditions, polymerization can be a reversible process. In this example, monomers are converted into polymers using a forward polymerization process. Notably, the process of depolymerization, which turns polymers back into monomers, is a complex one.
Why is polymerization chain reaction regarded as hazardous?
Polymerization is a chain reaction that is accelerated by the heat created throughout the process. The pressure and heat buildup may become uncontrollable, resulting in risks such as explosions or fires, among other things.
What is the reasons for Ethene's additional polymerization reaction?
Monomers must have double or triple bonds in order to participate in an extra reaction. Because free radicals are very reactive, they start the process. This free radical attacks the double bonds and carbon bonds in Ethene molecules, resulting in a chain reaction that produces polythene.









