Table of Contents
Surfactants are a type of chemical molecule that is used to reduce the surface tension (or interfacial tension) between various compounds, such as two liquids, a gas and a liquid, or a liquid and a solid. Surfactants are chemical molecules that are amphiphilic in nature. It has both hydrophobic and hydrophilic groups, which implies it contains both hydrophobic and hydrophilic groups.
- The water-insoluble group can migrate from the bulk water phase to the air or oil phase.
- In contrast, the water-soluble head group typically persists in the water phase.
Soaps are the most common surfactants, and they are derived mostly from fats known as glycerides, which are esters generated by trihydric alcohol, glycerol, and fatty acids with long-chain carboxylic acids. Glycerides are hydrolyzed by heating in a sodium hydroxide solution to produce soaps, sodium salts of acids, and propane 1,2,3 triol. This is known as saponification.
Surfactants: How Do They Work?
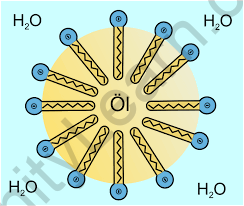
When a sufficient number of surfactant molecules are introduced to a solution, they begin to combine. In the bulk aqueous phase, they form structures or aggregates known as micelles. The surfactant heads (hydrophilic heads) stay exposed to water or the surrounding liquid while the micelle begins to form. The tails (hydrophilic heads) congregate in the structure’s centre and stay watertight. Aggregates of various shapes and sizes, such as spherical or cylindrical micelles or lipid bilayers, can be created. Furthermore, the chemical structure of the surfactants has a large influence on the morphology of the aggregates (balanced size between the hydrophilic head and hydrophobic tail).
Surfactants, in general, function by dissolving the contact between oils, water, and dirt. The oils and grime are also suspended, making them easier to remove.
Surfactant Action
- Surfactants are made up of hydrophobic (anti-water) and hydrophilic (water-loving) groups.
- Surfactant molecules are absorbed by the oil and hence removed from the surface.
- Surfactant molecules encircle the oil after it has been evacuated, preventing it from reaccumulating.
Manufacture
Surfactant glycerides comprise unsaturated and saturated carboxylic acids with an even number of carbon atoms in the range of 12-20, such as stearic acid. Synthetic surfactants have a significant advantage over soaps. Because soaps generate insoluble magnesium and calcium salts with magnesium and calcium ions in hard water and clays found in the soil, a lot of soap is wasted in the process of creating an insoluble mess. However, by employing a synthetic surfactant, this may be prevented.
FAQs
Surfactants Disinfect, do they have a Bad influence?
Disinfection and sterilization are suggested parts of healthcare to function in conjunction with other antimicrobial agents, such as surfactants and detergents, which loosen soils off surfaces. Surfactants are widely used in a variety of human activities due to their outstanding wetting and emulsifying properties. The effluent was dumped into the environment, damaging aquatic life, contaminating the water, and risking human health.
What Are Some Surfactant Examples?
Sodium stearate is a nice example of a surfactant. It is the most prevalent surfactant in soap. Another typical surfactant is 4-(5-dodecyl) benzenesulfonate.






