Table of Contents
Titration, also known as titrimetry, is a chemical qualitative analytical technique used to determine the concentration of a specific analyte in a mixture. Titration is an essential method in analytical chemistry, and it is also known as volumetric analysis.
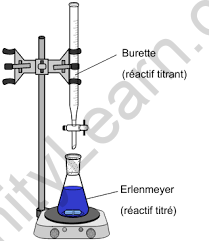
Titration Methodology
- Titration begins with the creation of a titrant/titrator, which is a standard solution with set volume and concentration. This titrant is then allowed to react with the analyte until an endpoint or equivalence point is reached, at which point the analyte concentration may be estimated by measuring the amount of titrant eaten. Titration, on the other hand, is a stoichiometric concept used to determine the unknown concentration of a solution.
- In terms of method stages, a highly exact quantity of analyte is introduced to a beaker or Erlenmeyer flask. A little quantity of the titrant (such as phenolphthalein) is put beneath a calibrated burette or chemical pipetting syringe containing the indicator.
- Small amounts of titrant are mixed with the analyte and indicator. This will continue until the indicator’s color changes in response to the titrant saturation threshold. At this moment, it will indicate that we have reached the conclusion of the titration. Essentially, the amount of titrant in this situation balances the amount of analyte present during the process.
Types of Titrations
Titrations are classed as follows based on the sorts of reactions involved.
- Acid-base Titration
- Redox Titrations
- Precipitation Titration
- Complexometric Titration
In some circumstances, the titrate may contain more than one component (for example, Na2CO3 + NaHCO3). Titrations may therefore be classified depending on the number of components in the titrate.
- Single Titration
- Double Titration
Titration Curve
The pH of the analyte solution vs the amount of titrant added as the titration advances is shown on a titration curve. The x-coordinate of a titration curve reflects the amount of titrant added since the start of the titration. The analyte concentration at the appropriate step of the titration is represented by the y-coordinate. The titration curve in an acid-base titration primarily indicates the strength of the matching acid and base.
FAQs
What is the distinction between an endpoint and an equivalence point?
The titration error is the difference between the endpoint and the equivalence point. The degree of titration error can be lowered by selecting an appropriate end-point signal and methods for detecting it. The indicator in an experimental process must be chosen carefully, taking into account the nature of the titration procedure. When the color of the indicator changes, the titration operation is complete. The endpoint of a titration procedure is used to compute the analyte concentration in relation to a known concentration of the titrant.
How do I choose the best indicator for acid-base titration?
An acid-base titration is an experimental technique used to determine whether a solution is acidic or basic. The indicators used to determine whether a solution is acidic or basic are typically weak acids or bases. Methyl orange is the most widely used indicator for predicting bases, whereas phenolphthalein is the most generally used sign for predicting acids.






