Table of Contents
The phenomenon exhibited by the nuclei of an atom as a result of nuclear instability is known as radioactivity. Henry Becquerel discovered this phenomenon in 1896. The process by which the nucleus of an unstable atom loses energy by emitting radiation is known as radioactivity. A small amount of Uranium compound was wrapped in black paper and stored in a drawer containing photographic plates. Following an examination of these plates, it was discovered that there had been an exposure. This phenomenon was dubbed Radioactive Decay. Radioactive elements are elements or isotopes that emit radiation and undergo radioactivity.
Because of the presence of an unstable nucleus in the element’s radioisotope, the atom particles cannot be bounded. The isotopes are constantly decaying in order to stabilise themselves, releasing a large amount of energy in the form of radiation. The process of converting an isotope into an element of a stable nucleus is known as transmutation.
It can happen both naturally and unnaturally.
He used visible light to illuminate some Uranium-Potassium-Sulphate fragments. Following that, he wrapped these pieces in black paper and separated them from a photographic plate with a piece of silver. He left it for a few hours. When he developed the photographic plate, he discovered that it had blackened. This meant that the compound emitted something that penetrated the silver and black paper and struck the plate. Subsequent experiments demonstrated that radioactivity is a nuclear phenomenon that occurs when an unstable nucleus decays. This is known as radioactive decay.
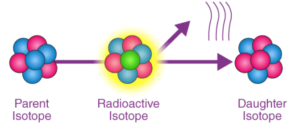
Types of Radioactive Decay
Radioactive decay is the process by which an unstable atomic nucleus loses energy by emitting radiation. There are three main types of radioactive decay: alpha decay, beta decay, and gamma decay.
Alpha decay is the process of emitting an alpha particle, which is a helium nucleus. In alpha decay, the nucleus loses two protons and two neutrons.
Beta decay is the process of emitting a beta particle, which is an electron. In beta decay, the nucleus loses one proton and one neutron.
Gamma decay is the process of emitting a gamma ray, which is a high-energy photon. In gamma decay, the nucleus loses energy without changing its atomic number or mass number.
In nature, there are three types of radioactive decays:
- Alpha
- Beta
- Gamma.
Law of Radioactive Decay
When a radioactive material decays, the number of nuclei decaying per unit time is proportional to the total number of nuclei in the sample material. So,
If N is the total number of nuclei in the sample and ΔN is the number of nuclei that decay in time Δt, then
ΔN/ Δt ∝ N
Or, ΔN/ Δt = λN … (1)
where λ denotes the radioactive decay or disintegration constant. The change in the number of nuclei in the sample is now given by dN = – ΔNin time Δt. As a result, the rate of change of N (in the limit Δt → 0) is,
dN/dt = – λN
Or, dN/N = – λ dt
Now, if we integrate both sides of the above equation, we get,
NN0∫ dN/N = λ tt0∫ dt … (2)
Or, ln N – ln N0 = – λ (t – t0) … (3)
Where N0 denotes the number of radioactive nuclei in the sample at any given time. t0 is the initial time, and N is the number of radioactive nuclei at any subsequent time t. After that, we set t0 = 0 and rearrange the above equation (3) to get,
ln (N/N0) = – λt
Or, N(t) = N0e– λt … (4)
Equation (4) is the Law of Radioactive Decay.
FAQs
What is the formula for the radioactive decay law?
If N is the size of a population of radioactive atoms at time t and dN is the amount by which the population decreases in time dt, the rate of change is given by the equation dN/dt = −λN, where λ is the decay constant.
What are the radioactivity laws?
All nuclei of a radioactive element have an equal chance of decaying. A radioactive element's rate of spontaneous disintegration is proportional to the number of nuclei present at the time. N: the number of atoms present at time t.: the element's decay constant.






