Linear Shape Of Molecules: The three-dimensional arrangement of atoms in space around a centre atom is known as a molecular shape. A substance’s molecular formula does not provide information on its shape. CO2, for example, is a linear molecule, but SO2 is angular.
The valence shell electron pair repulsion hypothesis may be used to predict the three-dimensional structures of many tiny compounds (VSEPR). When atoms join to create molecules, pairs of valence electrons are arranged as far apart as possible. Another technique to describe molecular form is using hybrid orbitals.
Linear molecular geometry
A linear molecule is one in which the atoms are arranged in a straight line (180° angle). Sp hybridization occurs at the centre atom of molecules with linear electron-pair geometries. Carbon dioxide (O=C=O) and beryllium hydride BeH2 are examples of linear electron pairs and molecular geometry.
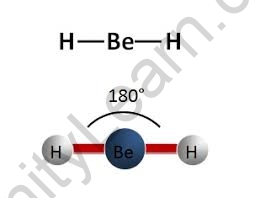
In chemistry, linear molecular geometry specifies the geometry centred on a central atom that is connected to two other atoms (or ligands) at a bond angle of 180°. Linear organic compounds, such as acetylene, are frequently characterised by using sp orbital hybridization to explain their carbon centres.
Linear geometry occurs at centre atoms with two linked atoms and zero or three lone pairs (AX2 or AX2E3) in the AXE notation, according to the VSEPR model (Valence Shell Electron Pair Repulsion model). Beryllium fluoride (FBeF) with two single bonds, carbon dioxide (O=C=O) with two double bonds, and hydrogen cyanide (HCN) with one single and one triple bond are examples of neutral AX2 molecules with linear geometry. Acetylene (HCCH) is the most important linear molecule with more than three atoms, with each carbon atom acting as a core atom with a single link to one hydrogen and a triple bond to the other carbon atom. Linear anions include azide and thiocyanate (SCN), while nitronium ion is a linear cation.
Also Read: Shapes of Molecules | Molecular Geometry – Linear, Trigonal Planar
FAQs on Linear Shape Of Molecules
Question 1.What are the benefits of VSEPR theory?
Answer: The following are the benefits of the VSEPR theory:
- The VSEPR hypothesis is particularly good in predicting the 3-D structure of molecules and ions.
- The VSEPR models are based on the assumption that electrons arranged around a central atom would organise themselves in such a way that repulsion is minimised, determining the geometry of the molecule.
- It can anticipate the form of almost all compounds with a central atom, but the central atom must not be metal.
Question 2. How Can the VSEPR Theory Be Used to Predict Molecule Shapes?
Answer: A molecule with only two atoms has a linear structure, according to the VSEPR Theory. One of the atoms in a three-atom or more-atom molecule is known as the central atom, and the other atoms are connected to it. If the core atom is attached to comparable atoms and is surrounded by only electron bond pairs, the repulsions between them result in an asymmetrical form of the molecule, and therefore regular geometry.
If it is connected to other atoms or is surrounded by a bond pair as well as a lone pair of electrons, the repulsion between them is comparable, resulting in deformed geometry. The molecule’s form is determined by the total number of electron pairs present surrounding the core atom.

