Table of Contents
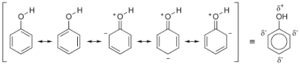 The Mesomeric effect refers to the polarity formed between atoms in a conjugated system by electron transfer or pi–bond electron transfer. In a nutshell, the mesomeric effect occurs when electrons in a conjugated orbital system move away from or towards a substituent group.
The Mesomeric effect refers to the polarity formed between atoms in a conjugated system by electron transfer or pi–bond electron transfer. In a nutshell, the mesomeric effect occurs when electrons in a conjugated orbital system move away from or towards a substituent group.
The mesomeric effect is classified into two types:
- +M effect
- -M effect
The +M effect (Positive mesomeric effect)
- When electrons or pi electrons are transported from one group to another, increasing the electron density of the conjugated system, this is referred to as the (+M) effect or positive mesomeric effect.
- For the +M effect to occur, the group must have either a lone pair of electrons or a negative charge.
- The +M effect gives the conjugate system a negative charge, alternatively, it may be argued that the electron density in the conjugate system rises as a result of this. These conjugate complexes are more reactive to electrophiles and less reactive to nucleophiles.
- Group exhibiting the +M effect: –NH, –NH2, –NHR, –NR2, –O, –OH, –OR, – F, – Cl, –O–COR, – NHCOR, –SH, –SR, and so forth.
The -M Effect (Negative mesomeric effect)
- The negative mesomeric (–M) effect occurs when pi-bond electrons are moved from the conjugate system to a specific group, resulting in a drop in the electron density of the conjugate system.
- For the –M effect to occur, the group must have either a positive charge or an unoccupied orbital.
- The –M effect increases the compound’s reactivity to a nucleophile by decreasing the electron density in the conjugate system while decreasing its reactivity to an electrophile for the same reasons.
- Among those who exhibit the –M effect are: –NO2, –CN, –COX, –SO3H, –CHO, –CONH2, –COR, –COOH, –COOR, and so on.
Mesomeric and Resonance Effects
Mesomeric and resonance effects are two important concepts in organic chemistry. Mesomeric effects are the delocalization of electron density within a molecule and resonance effects are the stabilization of a molecule due to the overlap of electron density between different parts of the molecule.
Mesomeric effects are due to the delocalization of electron density within a molecule. When a molecule has electron density that is delocalized, it is said to be resonance stabilized. This means that the molecule is more stable than it would be if the electron density was localized. The reason for this is that when the electron density is delocalized, it is spread out over a larger area. This makes the molecule less reactive because it is harder for an outside force to break the bond.
Resonance effects are the stabilization of a molecule due to the overlap of electron density between different parts of the molecule. When a molecule is resonance stabilized, it means that it is more stable than it would be if the electron density was localized. The reason for this is that when the electron density is delocalized, it is spread out over a larger area. This makes the molecule less reactive because it is harder for an outside force to break the bond.
Mesomeric effects and resonance effects are both important concepts in organic chemistry. Mesomeric effects are the delocalization of electron density within a molecule and resonance effects are the stabilization of a molecule due to the overlap of electron density between different parts of the molecule. These two concepts are important because they help to explain why certain molecules are more stable than others.
Resonance Characteristics
A resonance characteristic is a graph that shows how the impedance of a system changes with frequency. The impedance of a system is the measure of the opposition to the flow of current in an electrical circuit. The higher the impedance, the more opposition there is to the current.
There are several different types of resonance characteristics. The most common type is the impedance curve. The impedance curve shows how the impedance of a system changes with frequency. The impedance of a system is the measure of the opposition to the flow of current in an electrical circuit. The higher the impedance, the more opposition there is to the current.
The impedance curve is usually a sine wave. The sine wave represents the how the impedance varies with frequency. The amplitude of the sine wave is the impedance of the system at that frequency. The wavelength of the sine wave is the distance between two identical points on the curve.
The impedance curve is usually plotted on a logarithmic scale. This scale is used because the impedance of a system increases exponentially with frequency. The logarithmic scale makes it easier to see the changes in impedance over a wide range of frequencies.
There are also resonance curves. Resonance curves show how the impedance of a system changes with the applied voltage. The impedance of a system is the measure of the opposition to the flow of current in an electrical circuit. The higher the impedance, the more opposition there is to the current.
The impedance curve is usually a sine wave. The sine wave represents the how the impedance varies with the applied voltage. The amplitude of the sine wave is the impedance of the system at that voltage. The wavelength of the sine wave is the distance between two identical points on the curve.
The impedance curve is usually plotted on a logarithmic scale. This scale is used because the impedance of a system increases exponentially with voltage. The logarithmic scale makes it easier to see the changes in impedance over a wide range of voltages.
There are also resonance curves. Resonance curves show how the impedance of a system changes with the load. The impedance of a system is the measure of the opposition to the flow of current in an electrical circuit. The higher the impedance, the more opposition there is to the current.
The impedance curve is usually a sine wave. The sine wave represents the how the impedance varies with the load. The amplitude of
- Only electrons are delocalized in the resonance effect, not atoms.
- In all resonant structures, the number of lone pair electrons or unpaired electrons must be equal.
- All resonant structures must have the same amount of energy.
- This is a long-term consequence.
- Lewis structures must be followed by all resonant or canonical structures.
Energy of Resonance
The energy of resonance is a powerful force that can be harnessed and used for a variety of purposes. When two or more objects are in resonance with each other, their combined energy can be used to create powerful vibrations or oscillations that can be used to do work.
One example of the power of resonance is the way in which a tuning fork can be used to produce a musical tone. When a tuning fork is struck, it begins to vibrate at a specific frequency. If another tuning fork is placed nearby that is in resonance with the first, the second tuning fork will also begin to vibrate. The energy of resonance can be used to create powerful vibrations that can be used to produce sound waves.
Another example of the power of resonance is the way in which a bridge can be used to transfer energy. When a bridge is struck, it begins to vibrate at a specific frequency. If another bridge is placed nearby that is in resonance with the first, the second bridge will also begin to vibrate. The energy of resonance can be used to create powerful vibrations that can be used to transfer energy from one object to another.
FAQs
Q. What is the Importance of the Mesomeric Effect?
Ans: It specifies the charge distribution in the molecule and aids in determining the point at which electrophiles or nucleophiles attack.
Useful for defining physical properties like dipole moment and bond length.
Q. What are the four fundamental electron displacement effects?
Ans: There are four fundamental electron displacement effects.
- Inductive effect
- Electromeric effect
- Mesomeric effect
- Hyperconjugation effect






