Table of Contents
Introduction
Sulphur and oxygen-containing compounds like sulphur monoxide, sulphur dioxide, sulphur trioxide, and others are known as sulphur oxides. Sulphur dioxide and sulphur trioxide are the most common. Sulphur oxides are non-metallic compounds that react with water to produce an acidic solution.
A brief outline
Sulphur oxides form acid when they come into contact with water, hence they are acidic. Sulfur oxides are released into the atmosphere naturally by volcanic eruptions. It accounts for almost 67 per cent of all sulphur oxides on the planet. Human activity is responsible for the remaining 33% of CO2 in the atmosphere.
Important concepts
When sulphur-containing chemicals are burned in the air with ample oxygen, sulphur oxides are generated. It’s discovered in the burning of sulphide ores, the combustion of fossil fuels, coal, as well as other processes. Sulphur dioxide is produced naturally as a result of volcanic activity and as a by-product of copper metallurgy. Sulphur trioxide, on the other hand, is referred to as sulphuric anhydride since it is produced in industrial applications as a precursor to sulphuric acid. The lower sulphur oxides are generated as intermediates during the combustion of elemental sulphur, and they are less stable than SO2 and SO3.
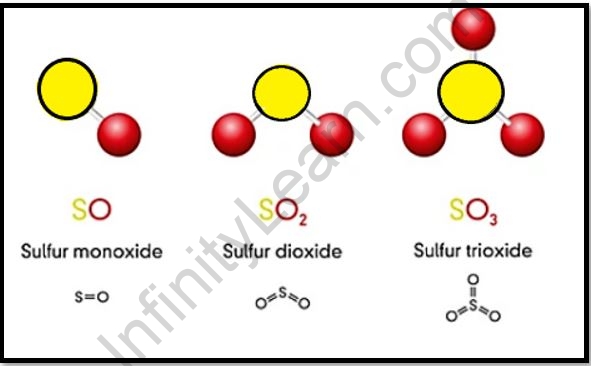
Important Oxides of Sulphur
While sulphur oxides come in a variety of forms, the two most common are:
- Sulfur dioxide (SO2)
- Sulfur trioxide (SO3)
- Sulfur dioxide (SO2)
Sulphur dioxide is one of the most frequent sulphur oxides on the planet and even in space. It’s a colourless gas that’s occasionally deadly and soluble in water.
- Sulfur trioxide (SO3)
When sulphur dioxide is oxidised, sulphur trioxide (SO3) is commonly generated. In a white crystalline solid, this chemical compound can be found in a variety of forms. It is colourless if it is in a liquid state.
Significance of oxides of sulphur in IIT JEE exam
The chapter on p block elements accounts for approximately 8.33 per cent of the total 120 marks on the JEE exam. The majority of the questions on this topic are answered using the NCERT textbook. There will certainly be a few questions concerning this topic in organic chemistry.
FAQs
What is the best way to get rid of sulphur?
The removal of sulphur from organic sulphur compounds is accomplished through a reaction with hydrogen, which results in the creation of hydrocarbon and hydrogen sulphide.
How Can Sulphur Be Removed from Fuels?
The treat gas is the gas that is used to eliminate sulphur from fuel. The treated gas is primarily made up of process by-products, or more specifically, hydrogen.
How can we cut the level of sulphur in our fuel?
A ship equipped with fuel can also use heavy fuel oil because the emission of superoxide is reduced, leaving an identical amount of fuel.








