Table of Contents
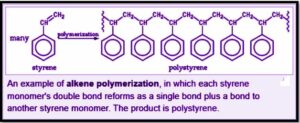
Introduction
Polymerization is the chemical reaction that links monomer molecules to produce molecular chains or three-dimensional structures. There are many multiple kinds of polymerization, and many classification schemes exist to distinguish them.
A brief outline
Based on functional groups contained in the reactants and their inherent steric effects, polymerization can occur in chemical compounds via a variety of reaction processes that vary in complexity. Alkenes produce polymers via relatively simple radical reactions in more straightforward polymerizations; however, processes involving replacement at a carbonyl group need more sophisticated synthesis due to the way reactants polymerize.
Important concepts
A polymerization could synthesize macromolecules with a straight or branched architecture. They can also come in the form of a three-dimensional complicated network. Step-growth polymerization, chain-growth polymerization (all of which fall under the category of addition polymerization), and condensation polymerization are among the most well-known types of polymerization reactions.
A polymer is a substance made up of very big molecules that are made up of many monomers, or repeating units. The process through which these monomers combine to generate the macromolecules that makeup polymers is known as polymerization.
The intricacy of the polymerization reaction process varies based on the functional groups present in the reacting monomer. Polymers are made from alkenes through the use of a free-radical action in the simplest polymerization. A polymerization process like this produces polyethylene, which is among the most economically valuable polymers.
Difference between Addition and Condensation Polymerization
Polymerization Types:
- Step-growth Polymerization: When pairs of reactants of any length fuse or combine at each step to generate a longer polymer molecule, this method is known as step-growth polymerization.
- Chain-growth Polymerization: When a step of chain extension adds to a monomer in a growing chain with an active centre such as a cation or a free radical, the polymerization is referred to as a Chain-growth polymerization since a chain reaction occurs.
Significance of polymerization in IIT JEE exam
Every facet of a chapter of a subject should be viewed as a whole. The most important chapter in chemistry is worth 13.3 percent of the grade, whereas the other chapters are worth 3.3 percent and 6.6 percent. Polymerization, which is covered in the polymer chapter, accounts for 3.3 percent of the exam and includes 1 to 2 questions.
FAQs:
Is polymerization reversible as a process?
In this example, monomers are converted to polymers by a forward polymerization process. Notably, the depolymerization process, which transforms polymers back into monomers, is a difficult one.
What makes the polymerization chain process so dangerous?
Polymerization is a chain of events that is accelerated by the heat created throughout the reaction. The pressure and heat build-up might spiral out of control, posing dangers such as explosions and fires.
What is the significance of Ethene undergoing a second polymerization reaction?
Because of its great reactivity, a free radical starts the reaction. These free radical attacks the double bonds and carbon bonds in Ethene molecules, causing a chain reaction to produce polythene.






