Table of Contents
Potassium dichromate, K2Cr2O7, is a common inorganic chemical reagent, most commonly used as an oxidizing agent in various laboratory and industrial applications. As with all hexavalent chromium compounds, it is acutely and chronically harmful to health. It is a crystalline ionic solid with a very bright, red-orange colour. The salt is popular in the laboratory because it is not deliquescent, in contrast to the more industrially relevant salt sodium dichromate.
STRUCTURE and MOLECULAR FORMULA OF POTASSIUM DICHROMATE
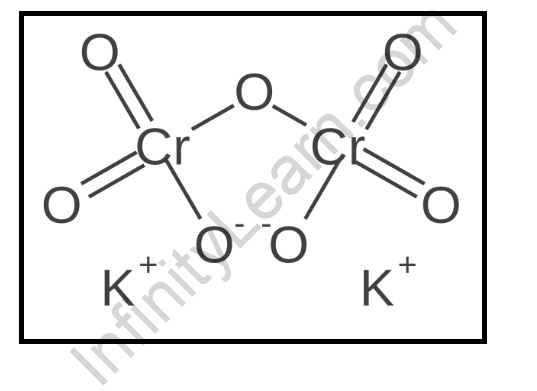
Potassium dichromate is an inorganic compound with the molecular formula K2Cr2O7. It is a salt of potassium ions and dichromate ions. It is a orange-red solid that is highly soluble in water.
The potassium ions are in the +1 oxidation state, and the dichromate ions are in the +6 oxidation state. The potassium ions and the dichromate ions are arranged in a square-planar geometry. The potassium ions and the dichromate ions are held together by ionic bonding.
The potassium ions and the dichromate ions are surrounded by six oxygen atoms. The potassium ions and the dichromate ions are also surrounded by six chlorine atoms. The potassium ions and the dichromate ions are held together by covalent bonding.
Occurrence
Potassium dichromate is an inorganic compound with the formula K2Cr2O7. It is a salt of potassium and dichromate, an oxidizing agent. It is a orange-red crystalline solid that is soluble in water. It is produced industrially by the oxidation of chromite ore.
Potassium dichromate is used in a variety of industries. It is used in the manufacture of dyes, pigments, and other chemicals. It is also used in the photography and textile industries. It is also used as a corrosion inhibitor and in the production of rubber.
Properties of Potassium Dichromate
Potassium dichromate has the appearance of red-orange crystals. Normally exist in a solid-state at room temperature.
It is odourless.
It is non-combustible and also its nature is highly corrosive.
The density of Potassium dichromate in the solid state is 2.676 g/cm3.
Its melting point is 398 °C (748 °F; 671 K) while its boiling point is 500 °C (932 °F; 773 K). It decomposes on boiling.
The solubility of potassium dichromate in the water at different temperatures is 4.9 g/100 mL at 0 °C and 13 g/100 mL at 20 °C and 102 g/100 mL at 100 °C which means it becomes highly soluble in water at higher temperatures. On the other hand, it is insoluble in alcohol, acetone.
The refractive index of potassium dichromate is 1.738.
Its crystalline structure is triclinic and the coordination geometry for the central atom chromium is tetrahedral.
FAQs
What is salt used for?
Potassium Dichromate is an Associate in Nursing odourless, orange to red, crystalline (sand-like) solid or powder. It's used as an Associate in Nursing analytical chemical agents and in tanning, painting, printing, electroplating, and pyrotechnics.
What is the distinction between KMnO4 and K2Cr2O7?
Potassium permanganate and salt area unit metallic element salts. The key distinction between permanganate of potash and salt is that permanganate of potash includes a dark violet colour, whereas salt includes a red-orange colour.
Where is K2Cr2O7 used?
Uses of salt (K2Cr2O7) - K2Cr2O7 is an Associate in Nursing oxidant for a range of reactions in laboratories and industries. It's employed in the animal skin trade for chrome tanning by acting as a precursor for metallic element mordant. It's employed in the volumetrical analysis. It's employed in colouring and calico printing.
Definition of dichromate?
An orange-to-red chromium salt usually contains the anion Cr2O72− dichromate of potassium. — also called bichromate.







