Table of Contents
Properties of Solids and Liquids: Liquids and solids diverge from gases in that the particles (atoms, molecules, or ions) are much closer together, resulting in a total volume much closer to the sum of the particle volumes. Atoms and molecules are in constant random motion at all temperatures above absolute zero. Unless they collide with another particle, the particles travel in a straight line. This molecular motion would cause all substances to be gaseous in the absence of any attractive forces. Solid and liquid states indicate the existence of forces that hold molecules and atoms together even when they are not chemically bonded. Intermolecular forces of attraction are the forces of attraction that hold atoms and molecules together in solid and liquid phases. Such forces differ from chemical bonds, referred to as intramolecular forces. A substance’s phase (solid, liquid, or gas) is the result of a competition between molecular motion, which pushes molecules apart, and attractive forces, which pull them together. The substance will be gaseous if the molecular motion is much greater than the attractive forces. The substance will be liquid if the molecular motion is nearly as strong as the attractive forces and solid if the molecular motion is much weaker than the attractive forces. Whenever the temperature is changed, the relationship between the molecular motion and the attractive forces changes, and the substance may change phase.
Overview
A solid is an accumulation of atoms or molecules that are held together in such a way that they maintain a defined shape and size under constant conditions. Solids are being classified into two types: crystalline and amorphous. Amorphous solids are disordered, whereas crystalline solids are well ordered at the atomic level. Crystalline solids are classified into four types: molecular solids, network solids, ionic solids, and metallic solids. Many of a solid’s macroscopic properties, such as electrical and heat conductivity, density, and solubility, are determined by its atomic-level structure and composition.
Liquids end up sharing some properties with solids (both are condensed matter and are relatively incompressible) and some with gases (such as their ability to flow and take the shape of their container). Majority of liquid properties, such as cohesion and adhesion, are influenced by intermolecular forces within the liquid. A compound’s viscosity is influenced by both its intermolecular forces and its molecular size. The majority of liquids we encounter in our daily lives are actually solutions, which are mixtures of a solid, liquid, or gas solute within a liquid solvent.
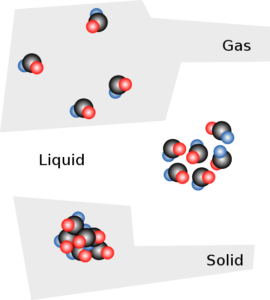
The Structure of Solids and Liquids
The molecular motion dominates in the gaseous phase. The substance’s molecules are completely separated and move independently of one another. Because the spaces between molecules are very large in comparison to the size of the particles, the volume of a gas is actually a measurement of the spaces between the molecules. In contrast, the liquid phase’s molecular structure has some spaces between the particles that allow them to move past one another, but the attraction between the particles is powerful enough here to keep them from drifting too far apart. The forces of attraction have completely overcome molecular motion in the solid phase, and particle movement has been reduced to vibrating in place. So, the particles are unable to move past one another and are held in a tightly packed pattern, leaving very little space between them.
Properties of Solids
As said, the intermolecular forces of attraction in solids grab the particles so tightly together that they cannot pull apart to expand their volume or flow past one another to change shape. As a result, regardless of their container, solids retain their shape and volume. Because there is very little empty space in the solid structure, solids are almost incompressible. Diffusion and mixing are essentially non-existent beyond the surface layer because molecules cannot pass through each other in the structure.
- Electrical and thermal conductivity:
Electricity has always been essentially the movement of electrons from one location to another, and the outer electrons in metallic bonds are relatively free to move between adjacent atoms. Because of this electron mobility, an electrical current can easily move from one end of a piece of metal to the other. When an electric current introduces an electron at one end of a piece of wire, electrons move from one metal atom to another continuously down the wire, allowing the current to flow. However, in other solids, the electrons are involved in covalent or ionic bonds and thus cannot or only weakly conduct electricity. Materials that don’t really conduct electricity are known as electrical insulators. Heat conductivity, as well known as thermal conductivity, is closely related to electrical conductivity. Metals are excellent electrical conductors, and you’re probably aware that they’re also excellent heat conductors. (This is why most kitchen pots, pans, and baking sheets are made of metal; they absorb heat from the stove or oven and transfer it to the food being cooked.)
- Malleability and ductility:
Malleability and ductility, two additional properties, exhibit similar trends to electrical and thermal conductivity. The ability to hammer a solid into a sheet without breaking it is referred to as malleability, and the ability to stretch a solid to form a wire is referred to as ductility. Metals, as you might expect, are malleable and ductile, owing to the non-directionality of metallic bonds. Covalent and ionic bonds, on the other hand, which are directional and require specific geometries resulting in fixed three-dimensional lattice structures, make many other types of solids brittle, causing them to break under force.
Metals’ malleability and ductility seem to be important reasons for their utility. Their conductance would be much less useful if they couldn’t be stretched into wires that could then be bent and shaped at room temperature for a wide range of applications. They do, however, have some drawbacks. Metal jewellery can become crushed and deformed in the bottom of a purse, and metal figurines can become dented if dropped. To find the best option for each application, manufacturers must consider all of the properties of the materials they intend to work with.
- Melting point:
Some other way to deform a solid is to melt it. The melting point of a solid is determined by the strength of the interactions between its constituents: A higher melting point is associated with stronger interactions. Melting a molecular solid means breaking the weak intermolecular forces (the forces that hold different molecules together), not the strong covalent bonds that hold the individual molecules together. The melting temperature of network solids (held together by covalent bonds), ionic solids (held together by ionic bonds), and metallic solids (held together by metallic bonds) depend on the specific bond strength of each solid.
- Solubility:
If we dissolve a solid, it will necessitate the breaking of various types of bonds for various solids. Dissolving a metal necessitates the breaking of metallic bonds, whereas dissolving a network solid necessitates the breaking of covalent bonds. Both of these types of bonds are extremely strong and difficult to break. As a result, metals and network solids are rarely soluble in water. (Diamond rings would probably be less valuable if the band and the stone disintegrated in the shower.) Dissolving a molecular solid, on the other hand, necessitates only the breaking of weak intermolecular forces, not the covalent bonds that hold the individual molecules together. As a result, molecular solids are relatively soluble, as you might expect given how much sugar we use in so many drinks.
- Density:
Another important property that depends on the structure and composition of the solid is density, which is defined as the amount of mass that exists in a given volume (for more information, see our Density module). It is indeed worth noting that, while we described the various types of crystal solids as having specific structural characteristics, there is significant variation within each type as well. Metallic solids, for example, do not all have the same atom arrangement.
Properties of Liquids
Attractive intermolecular interactions play a significant role in the behaviour of liquids. Liquids maintain their volume because their particles remain in contact with each other, but because the particles can flow past each other, liquids take the shape of their container. 100 mL of liquid will indeed be 100 mL in any container, but because liquid molecules are not held in a tightly packed pattern like solids, they can move past one another, allowing the liquid to conform to the shape of the container.
- Cohesion:
Oils are useful for these applications because they have low cohesion: the liquid molecules do not interact strongly with each other due to weak intermolecular forces. The principal intermolecular forces present in most oils and many other organic liquids – liquids primarily composed of carbon and hydrogen atoms, also known as non-polar liquids – are London dispersion forces, which are the weakest types of intermolecular forces for small molecules. Because of these weak forces, cohesion is low. Because the molecules do not interact strongly with one another, they can easily slide past one another.
- Adhesion:
Adhesion is a compound’s proclivity to interact with another compound. (By contrast, cohesion is a compound’s proclivity to interact with itself.) Adhesion explains how liquids interact with their containers as well as with other liquids.
- Viscosity:
The resistance of a fluid (liquid or gas) to a change in shape or movement of neighbouring portions relative to one another is described as viscosity and it is a measure of resistance to flow. Fluidity is defined as the reciprocal of viscosity and is a measure of the ease of flow. The resistance of a fluid (liquid or gas) to a change in shape or movement of neighbouring portions relative to one another is described as viscosity. Because a part of a fluid that is forced to move carries along adjacent parts to some extent, viscosity can be thought of as internal friction between molecules; such friction resists the development of velocity differences within a fluid. As far, viscosity is one of the major factor in determining the forces that must be overcome when fluids are used in lubrication and transported in pipelines. It regulates the flow of liquids in processes such as spraying and injection.
The atomic compositions, bonding, and structure of crystalline solids can differ. These characteristics, when combined, determine how different solids behave under different conditions. Conductivity, malleability, density, hardness, and optical transmission are just a few of the many properties of solids. A liquid is indeed a nearly incompressible fluid that conforms to the shape of its container while maintaining a (nearly) constant volume regardless of pressure. As being such, it is one of the four fundamental states of matter (the others being solid, gas, and plasma) and the only one with a fixed volume but no fixed shape.
FAQ’s
The shapes of salt and sugar do not appear to be fixed. What's the deal with it still being solid?
Sugar and salt are made up of tiny crystals. The shape of these crystals is fixed, so they are referred to as solids. Sugar and salt appear to take the shape of the container in which they are stored, but this is due to their small size; they do not change shape.
The shape of a rubber band changes. So, what is the significance of the term solid?
Rubber band turns change only when stretched (when a force is applied). Whenever the force is removed, it returns to its original shape. As a result, we can say that the rubber band has a fixed shape. It also satisfies all other solid properties (it has fixed volume, does not flow like liquids, and does not take the shape of a container).
What characteristics do liquids and gases have in common?
Liquids and gases seem to be similar in shape and volume because their shapes are determined by their surroundings.