Table of Contents
Rearrangement Reactions Of Alkyl Carbocation
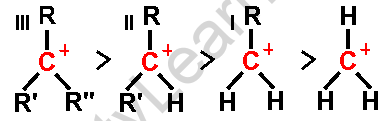
Carbocation rearrangements are prevalent in organic chemistry and are described as the transition of a carbocation from an unstable to a more stable state via different structural reorganizational “shifts” within the molecule.
When an alkyl halide, alcohol, or alkene is converted into a carbocation, it may undergo rearrangement. Carbocation rearrangements are classified into two types: hydride shifts and alkyl shifts. When the ensuing carbocation is rearranged, it will react further to generate a final product with a different alkyl skeleton than the initial material.
Reactions observed in Carbocations
- Carbocation reacts with nucleophilic compounds because they have a tendency to extract electrons to produce alcohol.
- Protons from carbocation are removed in this procedure to form alkene molecules.
- Carbocation undergoes a rearrangement reaction to generate more stable carbocation in this process.
The alkyl shift
- Not all carbocations contain acceptable hydrogen atoms (secondary or tertiary) on nearby carbon atoms that can be rearranged.
- In this situation, the reaction might undergo an alkyl shift, which is a distinct way of rearrangement (or alkyl group migration).
- The alkyl shift works similarly to the hydride shift.
- The electron pair carried by the moving alkyl group forms a bond with the surrounding carbocation.
- The moving alkyl group and the positive charge of the carbocation cause the molecule’s location to change.
- Tertiary carbocations are substantially more stable than primary or secondary carbocations, hence an alkyl shift on a primary or secondary carbocation to generate a tertiary carbocation occurs often.
Alkyl Shift Rearrangement Reaction Mechanism
- During the first phase of the rearrangement process, electrons align themselves with the C—-C bond. When a pair of electrons is given into the vacant p-orbitals, one C—-C bond begins to dissolve, forming a new C—-C bond.
- The pair of electrons from the C-C bond must align with the carbocation’s empty p orbital ( this means they have to be aligned in the same plane). Then, when the pair of electrons from the C–C bond is given into the vacant p-orbital, one C–C bond breaks, and a new C–C bond forms.
- In the transition state, partial bonds exist between the carbon being transferred and each of the two nearby carbon atoms. A tertiary carbocation is produced as an end product in the final phase of the reaction.
Any reaction that involves a carbocation intermediate is susceptible to rearrangement. During some electrophilic additions, carbocation rearrangements may occur.
FAQs
Why doesn't Rearrangement occur at 1 butyl Carbocation? What happens when 1butene interacts with HCl?
No rearrangement may occur immediately after the synthesis of the tertiary carbocation which will make it more stable.
Explain the concept of Alkyl Shift.
An alkyl shift occurs when a carbocation lacks a hydrogen atom that is present on the neighbouring carbon atom and is easily accessible or present to undergo a rearrangement process. It is also possible that the hybrid shift is unable to produce a stable carbocation. In this scenario, an alkyl shift is used.
Can both reduction and rearrangement processes happen at the same time?
No







