Solubility is the quantity of material (ionic or otherwise) soluble in a certain volume of solvent to form a saturated solution at a given temperature and volume of solvent. Solubility varies depending on the type of the material, the solvent, the temperature, and the presence of common ions.
- Water has an extremely low solubility of sparingly soluble compounds (AgCl, BaSO4). Despite their limited solubility, ionic substances can be regarded to create a saturated solution in which the ions are in equilibrium with the undissolved solid.
- Solubility product constants are used to characterize saturated solutions of ionic compounds with limited solubility. In a saturated solution, the dissolved, dissociated ionic component and the undissolved solid are in dynamic equilibrium.
The solubility product constant (Ksp) represents the equilibrium in a solution between a solid and its component ions. The constant’s value indicates the degree to which the chemical may dissociate in water. The higher the Ksp, for example, the more soluble the chemical. Because Ksq is a measure of a concentration that depends on conditions such as temperature, pressure, and composition, it is defined in terms of activity rather than concentration. It is impacted by the environment. The term Ksp is used to characterize an ionic compound’s saturated solution. (A saturated solution is one in which the dissolved, dissociated, undissolved solid and the ionic component are in balance.)
Most insoluble ionic chemicals will nevertheless dissolve in water to some extent. Because whatever fraction of the chemical is dissolved also dissociates, these insoluble compounds are termed strong electrolytes.
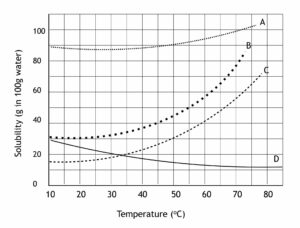
Relationship between Ksp and Solubility
- Solubility and solubility product constants are related in that one can be used to calculate the other. In other words, there is a link between the molarity of the solute and the ion solubility since Ksp is the product of the ion solubility in moles per litre.
- To calculate the Ksp of a slightly soluble molecule from its solubility, for example, the solubility of each ion must be transformed from mass per volume to moles per liter in order to calculate the molarity of each ion. These quantities may then be entered into the Ksp formula, which is the product of each ion’s solubility.
The Significance of Solubility Product
- Several factors influence the solute’s solubility in a particular solvent.
The presence of a common ion can influence equilibrium and hence concentration (solubility), but not the solubility product. - When the ionic product surpasses the Ksp, the solutes precipitate.
FAQ’s
Q. What is the importance of the Solubility product?
ANS: The following are the implications of the solubility product:
- The magnitude of a substance’s solubility product determines its solubility.
- The solubility product is a heterogeneous equilibrium constant or a particular version of the equilibrium constant. It is used in saturated solutions when an ionic component has not been entirely dissolved.
- Because temperature affects solubility products, the temperature at which they were measured must always be given.
Q. What are the applications of Ksp?
ANS: The following are some of the applications of Ksp.
- The magnitude of a substance’s solubility product determines its solubility.
- When two solutions are mixed, the solubility product can be used to predict the formation of a precipitate.




