Table of Contents
Interhalogen compounds are compounds that are formed when elements of the halogen group react with each other. In other words, it is a molecule consisting of two or more different elements of group 17. There are four types of interhalogen compounds:
Diatomic Interhalogen (AX)
Tetratomic Interhalogen (AX3)
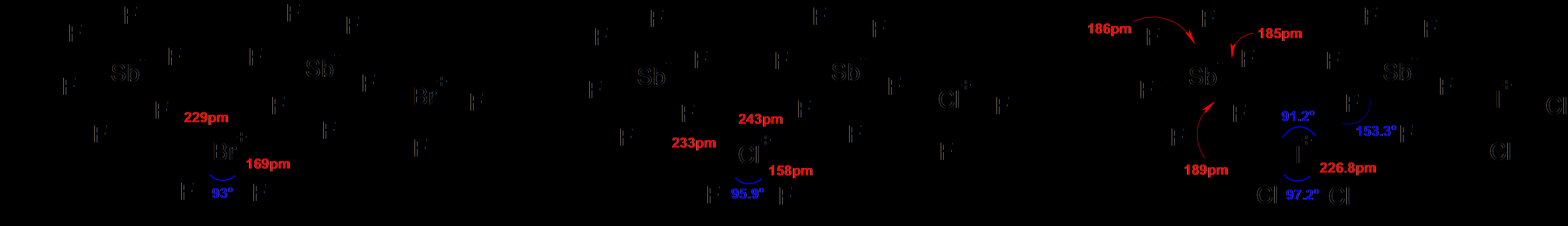
Hexatomic Interhalogen (AX5)
Octaatomic Interhalogen (AX7)
The halogen group with larger size and higher electropositivity reacts with an element of 17 with smaller size and lower electropositivity. As the ratio of the radii of the larger and smaller halogens increases, the number of atoms in the molecule also increases.
Preparation of interhalogen compounds
These molecules are formed either by direct combination or by the action of group 17 elements containing fewer interhalogen compounds under specific conditions. For example: At 437K, chlorine reacts with an equal amount of fluorine to form ClF. This method is widely used in the production of group 17 fluoride.
Cl2 +F2
2ClF (473K)
I2 + Cl2
2ICl
Properties of Interhalogen Compounds
- These molecules are covalent and diamagnetic in nature.
- The bonds formed between these compounds are more reactive than the diatomic halogen bonds.
- The physical properties of these molecules are transitional between their components.
- The molecular structure of AX3 molecules is curved T shape, AX5 molecules are square, or pyramidal and AX7 has a bipyramidal or pentagonal structure.
- The bond length depends on the size of the constituent halogens.
- The molecule that contains the lighter group 17 elements is quite colourless, but the one that is composed of higher halogens is darker in colour, which is due to the increase in molecular weight.
Uses of Interhalogen Compounds
These are used as non-aqueous solvents.
They are used as catalysts in some reactions.
UF6, which is used in the enrichment of 235 U is produced using ClF3 and BrF3.
These are used as fluorinating compounds.
FAQs
Explain pseudohalogenation?
Polyatomic analogue halogens are also known as pseudo halogens. Is that their chemistry is very similar to that of true halogens, but it also allows them to substitute for halogens in many classes of chemical compounds. Cyanide, cyanate, thiocyanate and some of the well-known pseudo-halogens reside in functional groups.
Can Fluorine Ever Be a Central Atom? Why Can’t Hydrogen Be the Central Atom?
Fluorine can't be a central particle in interhalogen compounds. this is often because it's a part of the second cycle of the table . Since it's 7 valence electrons, it can only form one bond. Hydrogen isn't the central atom. we will attribute this to the very fact that the atom will always attempt to achieve the foremost minimal energy. within the case of hydrogen, this suggests that it can only form one bond. It also features a very small size and doesn't fit into the opposite molecules around it.






