Table of Contents
Uses of Sulphur Dioxide
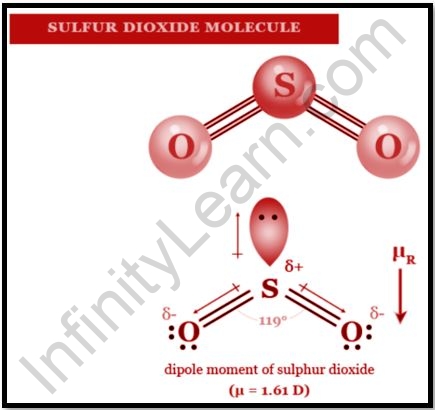
The synthetic compound sulphur dioxide, regularly known as sulphur dioxide, has the equation SO2. It’s a toxic gas that radiates the scent of consumed matches. It is made as a side-effect of copper extraction and the ignition of sulphur-bearing petroleum products and is delivered normally by volcanic movement. The smell of sulphur dioxide is like that of nitric corrosive. SO2 is a C2v evenness point bunch bowed particle. The holding would be depicted as far as reverberation between two reverberation structures utilizing a valence bond hypothesis approach utilizing just s and p orbitals. The bond request of the sulphur-oxygen bond is 1.5. This essential procedure, which doesn’t need d orbital cooperation, has some help. The sulphur particle has an electron include of one in electron counting formalism. Let us learn about the uses of sulphur dioxide in this article.
| S.NO | CONTENT |
| 1 | INTRODUCTION |
| 2 | A BRIEF OUTLINE |
| 3 | IMPORTANT CONCEPTS |
| 4 | SULPHUR DIOXIDE EXAMPLES |
| 5 | FAQs |
A Brief Outline
Before we learn about the uses of Sulphur Dioxide, let us understand what it is and its properties. Sulphur dioxide can be found in varying proportions on other planets, the most notable of which is the environment of Venus, where it is the 3 abundant atmospheric gas at 150 parts per million. It combines with water to generate sulphuric acid clouds, which is an important part of the planet’s global sulphuric cycle and causes global warming. Since it only exists in trace amounts, it has been identified as a crucial factor in the warming of early Mars, with predictions of concentrations in the lower atmosphere as high as 100 ppm. Its major source is assumed to be volcanic on both Mars and Venus, as it is on Earth.
Sulphur dioxide is an extreme air foreign substance that has genuine well-being outcomes. Besides, how much sulphur dioxide in the climate affects the appropriateness of the environment for plant gatherings and creature life. Sulphur dioxide discharges are forerunners of corrosive downpours and particles in the air. For coal-terminated power plants, many innovations with generally high productivity have been created to restrict sulphur discharges. By utilizing limestone as bed material in fluidized bed burning, sulphur can be dispensed with from coal during ignition.
Important Concepts
Uses of Sulphur dioxide gas
- Sulphur dioxide is a poisonous gas that causes a variety of health concerns, but we use it to make industrial products like sulphur acid, sulphur trioxide, and sulphites. Steel, fertilizers, medications, fuels, batteries, paper, plastics, and other products are made with sulphuric acid, which is obtained from sulphur dioxide.
- It is used to remove excess chlorine as a disinfectant or bleaching agent.
- Food preservation uses liquid sulphur dioxide as a refrigerant.
- In the laboratory, it is utilized as a reducing agent or solvent for a variety of chemical processes.
- It is commonly employed in the development of pest control products due to its toxicity.
Sulphur dioxide examples
Sulphuric acid precursor
Because sulphur dioxide is changed to sulphur trioxide and then to oleum, which is eventually converted to sulphur acid, it is a precursor of sulphuric acid. When sulphur reacts with oxygen to perform this function, sulphur dioxide is produced. Sulphur dioxide is converted to sulphuric acid through the contact process. Several billion kg are produced each year for this purpose.
Sulphuric acid as a reducing agent
Sulphur dioxide also operates well as a reduction agent. Sulphur dioxide has the potential to decolourize things in the contact with water. It’s a good reducing bleach for papers as well as other sensitive things like textiles. Usually, the bleaching effect will not last too long.
Sulphuric acid as biomedicine
A biological intermediate is sulphur dioxide or its conjugate base bisulphite, which is produced by both sulphate-reducing organisms and sulphur-oxidizing bacteria. The role of sulphur dioxide in mammalian life is not well understood. When sulphur dioxide suppresses neuronal impulses from the pulmonary stretch receptors, the Hering–Breuer inflation reflex is inhibited.
Endogenous sulphur dioxide would be thought to make a significant contribution in regulating cardiac and carotid artery function, and abnormal or insufficient sulphur dioxide pathway has been linked to a wide range of cardiovascular diseases, including hypertension, atherosclerosis, pulmonary hypertension, and stenocardia.
Sulphuric acid as reagent and solvent
- Sulphur dioxide is an innocuous solvent that is frequently used to break down highly oxidizing compounds. It’s also exploited as a sulphonyl group source in organic synthesis occasionally.
- It functions as preservation.
The major uses of sulphur dioxide
- Sulphur dioxide is used as a postharvest fungicide and preservation for grapes.
- Sulphur dioxide or sulphites added to dehydrated vegetables extends their storage life, preserve colour and flavour, and aid in the conservation of ascorbic acid and carotene.
- Bleaching textile fibres, straw, wicker ware, gelatin, glue, and beet sugars; bactericidal in breweries and food industries; bleaching textile fibres, straw, wicker ware, gelatin, glue, and beet sugar content
Make your IIT Dream come true with Infinity Learn.
FAQs
Sulphur dioxide, or SO2, is a colourless gas or liquid with a pungent scent. It comes from the smelting of sulphur-containing mineral ores and the burning of fossil fuels (coal and oil) (aluminium, copper, zinc, lead, and iron). In water, sulphur dioxide dissolves fast to generate sulphuric acid.
Sulphur dioxide is an acid gas, which may be easily proved by filling a gas container with water and a few drops of universal indicator. Sulphurous acid (H2SO3) is formed, which is a slightly dibasic acid. What causes sulphur dioxide to form?
Is sulphur dioxide an acid or a base?
Infinity Learn App
Now you can find answers to all your subject queries & prepare for your Exams on our Educational App – Infinity Learn.







