Table of Contents
Introduction:
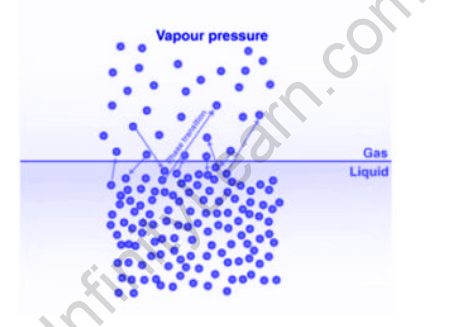
All fluids show a propensity for vanishing. The course of dissipation happens at the outer layer of the fluid. Assuming the dynamic energy of fluid particles conquers the intermolecular power of fascination in the fluid state, then, at that point, the atoms from the outer layer of the fluid break into space over the surface. The cycle is called ‘dissipation.’ If vanishing is done in a shut compartment framework, then, at that point, the fumes of fluid stay in touch with the fluid’s surface.
Like gas particles, the buildup of atoms likewise executes the consistent irregular movement. During these movements, particles crash into one another and, even with the dividers of the compartment, they lose their energy and return to the fluid state. This cycle is called ‘condensation.’
Dissipation And Condensation
- Dissipation and buildup are persistent cycles. Henceforth, after some time, a balance is laid out at a steady temperature among dissipation and buildup. At the balance number of particles in the fume, the state stays stable at a steady temperature.
- The fluid’s fume pressure relies upon the idea of the liquid and temperature, with an increment of intermolecular power of fascination Vapor tension of fluid reductions, and with a climbing temperature fume strain of fluid increments. A mercury manometer might be utilized to decide the fume tension of a fluid.
What is vapour pressure?
- Fume pressure is the propensity of a material to change into a vaporous state. Also, by and large, fume pressure is the tension applied by the fume of the fluid in Thermodynamic harmony with the consolidated stages in a shut framework. On the off chance that the temperature of any fluid expands, its fume pressure additionally increments. The fume tension of a fluid can be estimated in different ways. The least difficult technique to gauge fume pressure is involving a manometer in a shut holder or cup.
Factors Affecting Vapor Pressure
There are different variables on which fume pressure depends. They are:
1. Nature of Liquid
The idea of the fluid is made sense of in light of its intermolecular powers. In other words, as the size of the intermolecular powers ascend, the fume strain will decrease down
2. Impact of Temperature
As the temperature of the fluid expands, the dynamic energy related to the fluid likewise increments. What’s more, because of this expansion in motor energy, the getting away from the inclination of the particle increments; henceforth fume pressure increments. So we can draw the induction that fume pressure is straightforwardly corresponding to temperature.
3. Centralization of Solute
The presence of a solute in the fluid will fundamentally decrease the fume pressure. What’s more, this fall in fume pressure additionally contrasts concerning the convergence of solute.
4. Vapour Pressure Is Independent of Humidity.
Temperature is the main property that influences the fume tension for a specific measure of water fume in the air. Dampness will act provided that the wide range of various factors are steady. So get no kind of disarray between the impact of temperature and dampness.
5. Volume
Fume pressure won’t generally get impacted by the volume of the holder. As we probably are aware, that fluid in this case will be in harmony with the fume Now, assume the volume of surface S is isolated into limitless rudimentary volumes to such an extent that volume is changed, say diminished, then a portion of the compartment’s fume transforms into a fluid state. Also, assuming the volume ascends, a portion of the fluid will undoubtedly change into its fume state.
6. Surface Area
- Normally, fume pressure is autonomous of the surface region.
- Factors Affecting Vapor Pressure of Liquid:
Be that as it may, the accompanying variables influence the fume strain of fluid at balance.
Intermolecular Forces of Attraction
The powers that intervene in a connection between particles, including powers of fascination or shock are known as the Intermolecular powers (IMF). For instance, the covalent bond, including sharing electron sets between particles, is a lot more grounded than the gatherings intermolecular power present between adjoining atoms.
The Volume of the Liquid Present Does Not Affect the Vapor Pressure of a Liquid at Equilibrium.
We can change the volume of fluid (keeping temperature steady), however, the fume strain of a liquid at balance will continue as before.
The Temperature of the Liquid.
More fragile are the intermolecular powers of fascination, or higher is the temperature of the fluid, higher is the fume tension of a liquid at harmony.
Unit of Vapor Pressure
- The most well-known unit for fume pressure is the torr. One torr = 1 mm Hg (one millimetre of mercury).
- Most materials have outer fume pressures. For instance, water has a fume tension of roughly 20 torrs at room temperature (22 °C = 72 °F). Note that the fume pressure increments with the temperature; water will have a fume tension of 760 torr = 1 atm at its edge of boiling over of 100 oC (212 oF).
Boiling point
- The edge of boiling over alludes to the temperature of any substance at which the fume tension of fluid becomes identical to the strain incorporating the fluid, and the liquid believers into a fume.
Connection Between the Standard Boiling Point And The Vapor Pressure of Liquids
- The higher the fume strain of a fluid at a given temperature, the lower the standard limit (i.e., the edge of boiling over at environmental tension) of the fluid.
- The fume pressure outline to one side has charts of the fume pressures versus temperatures for an assortment of fluids.
- As should be visible on the guide, the fluids with the most noteworthy fume pressures have the least standard limits.
For instance, at some random temperature, methyl chloride has the most elevated fume tension of any of the fluids in the graph. It additionally has the least standard limit (−24.2 °C), which is the place where the fume pressure bend of methyl chloride (the blue line) converges the flat strain line of one environment (atm) of outright fume pressure.
FAQs
What is the boiling point?
An edge of boiling over is the temperature at which fluid begins to bubble and transforms into a fume or vaporous state. At this temperature, the tension applied by environmental factors on the fluid is equivalent to that of strain applied by the fume by the fluid. Fume tension will be high in the event that the intermolecular powers of the particles are low. This implies next to no energy will be expected to isolate the particles and thus the limit will likewise be moderately low.
What is Raoult's law?
Raoult's regulation is a regulation that expresses that the freezing and limits of an ideal arrangement are discouraged or raised relying on the unadulterated dissolvable. Raoult's regulation likewise expresses that the fume strain of the fluid arrangement is relative to the mole part of the dissolvable. This regulation assists with inferring the fume tension of the fluids.
What are the elements influencing vapour pressure?
Vapour strain of any fluid is reliant upon certain elements which are surface region, kind of atoms, and temperature. Assuming the temperature increments, fume pressure likewise increments, and then again on the off chance that temperature diminishes, fume pressure diminishes. Concerning the surface region, fume pressure has no impact on a superficial level region of the strong or fluid which is in touch with the gas. Also, in the event that the intermolecular powers between the atoms are solid, the fume pressure is generally low, though, on the off chance that the intermolecular powers between the particles are somewhat feeble, fume pressure is moderately high.