Table of Contents
The iron cardon phase diagram is like a picture showing the different forms a substance can take at different temperatures, pressures, or amounts. Knowing about the Iron (Fe) – Iron Carbide (Fe3C) phase diagram is really useful in metallurgy and materials science. It helps us figure out how steel and other iron-based metals will look and behave.
In this article, we’re going to talk about the Iron-Iron Carbide phase diagram in simple terms. We’ll cover what the diagram shows, the different reactions that happen in the metal, why it’s important, and give a few examples to help understand it better.
Also Check: Autoclave Diagram with Label
Iron Carbon Diagram
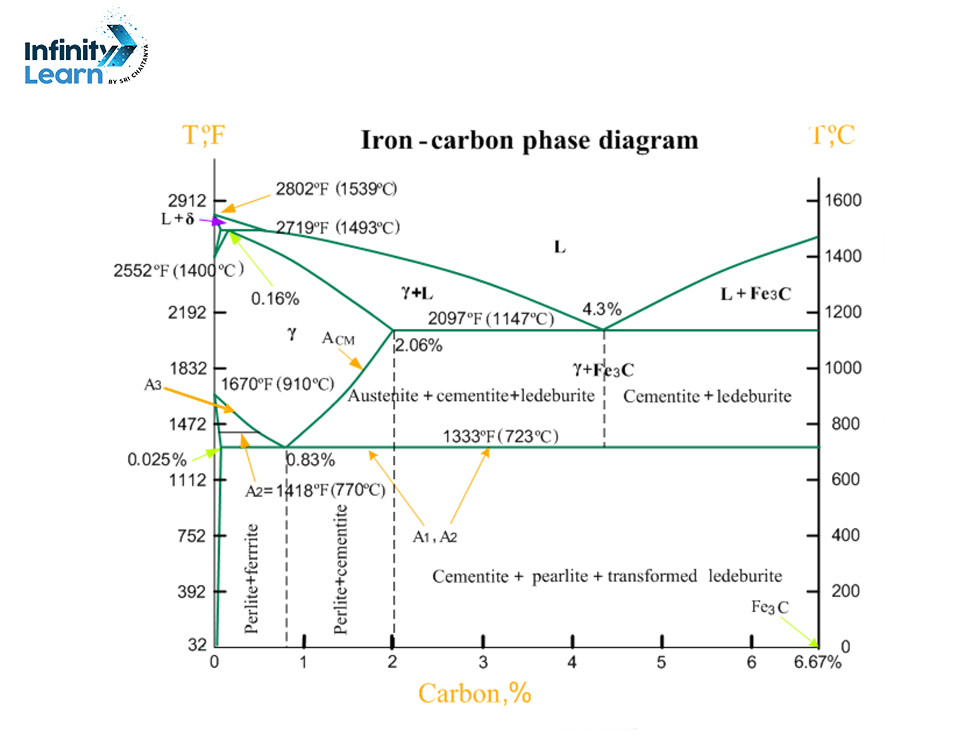
What Are Phase Diagrams?
Phase diagrams are pictures that show what phases are in a mix at different temperatures, pressures, or chemical mixes.
These diagrams tell us when two or more phases can be together in balance. For instance, the water phase diagram shows a spot (called the triple point) where water can be in three different phases all at once. This happens at a bit above freezing (0.01°C) and low pressure (0.006 atm).
What Are the Different Phases in the Iron Carbon Phase Diagram?
α-ferrite
Alpha ferrite is a phase of iron and carbon that forms when a low concentration of carbon cools down from a liquid state. It happens during the cooling process when the liquid turns into austenite. Having alpha ferrite in iron-carbon mixtures helps prevent damage to the structure, slows down the growth of grains, reduces susceptibility to fatigue, and increases strength.
δ-ferrite
Delta ferrite is a type of iron with very little carbon in it, which gives it a certain crystal structure. When more carbon is added, it mixes with another substance called cementite, forming a combination of delta ferrite and cementite. Delta ferrite remains stable up to a temperature of 912 degrees Celsius. Beyond this temperature, it changes into another type of structure called austenite. Delta ferrite is magnetic and flexible but not very strong.
γ-austenite
Gamma austenite is a solid phase of iron with a particular crystal structure that remains stable until it reaches a high temperature of 1,395 degrees Celsius. At this point, it changes into a different structure called ferrite. Many iron alloys that undergo heat treatment start off in the gamma austenite phase. Gamma austenite is not magnetic, and it’s soft and flexible.
Martensite
Martensite is created when austenite is rapidly cooled down. This quick cooling process is called martensitic transformation. Martensite is very hard and strong, achieved through a process called quenching.
Fe3C (Cementite)
Fe3C, also known as cementite, is a chemical compound formed when there’s carbon in iron, starting from any amount above zero, but fully forming when there’s 7% carbon content. It can be made by cooling down austenite or tempering martensite. Cementite is hard but also brittle. One of its best features is its ability to resist corrosion, especially when treated with a solution containing 1-3% sodium chloride.
Ledeburite
Ledeburite is a mix of two substances: austenite and cementite, with a carbon content of 4.3%. This carbon content is too high for steel but suitable for cast iron. Ledeburite melts at a temperature of 1,147 degrees Celsius.
Pearlite
Pearlite forms when an iron-carbon mixture cools down slowly. It consists of layers of two substances: ferrite and cementite. This combination gives pearlite toughness and strength.
What Are the Different Reactions in the Iron Carbon Phase Diagram?
The microstructure of iron-carbon mixtures is influenced by two main things: temperature and how much carbon is present. When you heat or cool these mixtures, the way the iron and carbon atoms bond together changes. Let’s talk about three types of bonding:
Peritectic: This is when a liquid and a solid react to form another solid. It’s not as common as eutectoid or eutectic reactions, and there’s only one type of this reaction in iron-carbon mixtures.
Eutectoid: This happens when, at a specific point called the eutectoid point, one type of solid turns into two different solids at the same time while cooling down. For example, in iron-carbon mixtures, this occurs at 0.8% carbon and 723°C. Here, austenite changes into ferrite and cementite.
Eutectic: Similar to eutectoid, but it starts with a liquid turning into two solid materials as it cools. This happens at the mixture’s melting point, which varies depending on the mix.
Also Check: Block Diagram of Computer System
What Are the Types of Steel Used in the Iron Carbon Phase Diagram?
Tough Steel
Tough steel has less carbon, about 0.30–0.60%. It’s a good middle ground between soft and hard steel. Though it’s stronger than soft steel, it usually needs a special cooling process to make it really tough. Tough steel is commonly used for things like strong containers, gears, shafts, axles, and machine parts.
Hard Steel
Hard steel, also called carbon tool steel, has a lot of carbon in it, around 0.60–1.50%. Because of this, it’s tough and strong, but not very bendy. It can resist rust pretty well too, unless there are other metals mixed in, like chromium. Hard steel is used for sharp tools, molds, springs, and strong wires.
Soft Steel (or Regular Steel)
Soft steel has the least amount of carbon, only about 0.05–0.30%. It’s known for being easy to shape and bend. Because it doesn’t have much carbon, it’s also the cheapest and most common type of steel used for everyday stuff like screws, building frames, bridges, car bodies, and pipes.
Iron Carbon Phase Diagram FAQ’s
What is an iron carbon diagram?
It's a chart showing how carbon and iron combine to form different structures at different temperatures.
What is the structure of carbon and iron?
Carbon can form different structures like graphite and diamond, while iron can form structures like ferrite and cementite.
What is pearlite in an iron carbon diagram?
Pearlite is a mixture of ferrite and cementite, forming a layered structure.
What is the significance of iron-iron carbide diagram?
It helps understand the phases iron and carbon go through at different temperatures, important in metallurgy.
Why is 6.67% C present in an iron carbon diagram?
This percentage represents the maximum solubility of carbon in iron at a specific temperature.
What is the iron-iron carbide diagram reaction?
It shows the transformation of iron and carbon into different phases like ferrite, cementite, and pearlite.
How many invariant reactions are in an iron-carbon diagram?
There are three invariant reactions in the iron-carbon diagram.
What is the chemical reaction of iron-carbon?
It involves the formation of different phases like austenite, ferrite, and cementite at specific temperatures.
What is eutectoid in iron carbon diagram?
Eutectoid is the composition where austenite transforms into pearlite upon cooling.
What is the eutectoid reaction in the Fe-C system?
It's the reaction where austenite transforms into pearlite at a specific temperature.
What is the eutectic reaction in the iron carbon diagram?
It's the reaction where liquid phase transforms into a mixture of austenite and cementite at a specific temperature.









