Table of Contents

Sweeteners are food additives used to reduce the number of calories in a meal. Sugar comes from sugarcane or sugar beets and is a natural sweetener. Artificial sugar substitutes give food a similar sweetness to sugar but fewer calories. Saccharine, aspartame, neotame, acesulfame K, stevia, and sucralose are the most prevalent artificial sweeteners approved by the FDA. Different processed goods, such as bread items, jams and jellies, tinned food puddings, and soft drinks, contain these sweeteners.
Some common examples of artificial sweetening agents include Saccharin, Aspartame, Sucralose, & Alitame.
How does an artificial sweetener work?
For a sweetening agent to act correctly, it must be water-soluble. It should also easily bind to a receptor molecule found on the tongue’s surface. The receptor is physically linked to a G-protein. When the sweetener attaches to the receptor, the G- protein begins to dissociate. This activates a neighbouring enzyme, which sets in motion a series of events that lead to the signals being relayed to and interpreted by the brain. The sweetness of an artificial sweetening agent is revealed by the interaction between the receptor and the sweetener.
Saccharin
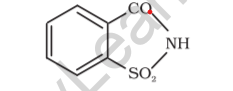
Saccharin is a sweetener that is either non-nutritive or artificial. It’s created in a lab by oxidizing o-toluene sulfonamide or phthalic anhydride. It seems to be a white crystalline powder.
Saccharin is a sugar replacement that contains neither calories nor carbohydrates. Saccharin cannot be broken down by humans, therefore, it remains unmodified in your body.
Because it’s 300–400 times sweeter than ordinary sugar, a small amount is all you need to get a sweet taste. It can, however, leave an unpleasant and harsh aftertaste. Saccharin is frequently combined with other low- or zero-calorie sweeteners because of this.
Saccharin, for example, is occasionally mixed with aspartame, a low-calorie sweetener used in carbonated diet drinks.
Saccharin is commonly used in food since it is quite stable and has a long shelf life. Even after years of preservation, it is safe to eat.
Saccharin is used to sweeten low-calorie candies, jams, jellies, and cookies, in addition to carbonated diet drinks. It’s also found in a variety of pharmaceuticals.
Saccharin can be sprinkled on food like cereal or fruit, or used as a sugar substitute in coffee or baking, just like table sugar.
Saccharin is a sweetener that can be found in a range of diet foods and beverages. It’s also used as a sweetener at meals. Sweet ‘N Low, Sweet Twin, and Necta Sweet are some of the brands that sell it. Saccharin comes in granular or liquid form, and one serving is equivalent to two tablespoons of sugar in sweetness.
Artificially sweetened drinks are another major source of saccharin, however, the FDA limits the quantity to no more than 12 mg per fluid ounce. Because saccharin was banned in the 1970s, many diet drink companies resorted to aspartame as a sweetener, which they still use today.
Baked foods, jams, jelly, chewing gum, canned fruit, confectionery, dessert toppings, and salad dressings all contain saccharin.
It’s also found in toothpaste and mouthwash, among other cosmetics. It’s also found in a variety of medicines, vitamins, and pharmaceuticals.
Saccharin that has been added to food or drinks in the European Union is labelled as E954 on the nutrition label.
Use of Saccharin
Saccharin comes in the form of acid as well as a calcium- or sodium salt, which is employed in the food industry, primarily in the salt industry, due to its high water solubility. The sweetness of the various forms is not significantly different; the level of sweetness for each derivative varies between 300 and 800 times that of sucrose and is depending on concentration and other parameters. In comparison to a sucrose solution, there is an inverse relationship between sweetening intensity and concentration, as with most other sweeteners. A 1 per cent saccharin solution, for example, is approximately eight hundred times sweeter than a sucrose solution, but a 3 per cent sucrose solution is just five hundred times sweeter.
Saccharin’s sweetening power is determined by its concentration as well as the composition of the solution. More than the additive values of each component, a combination of saccharin and other sweeteners can produce a significant amount of sweetening intensity. Furthermore, combining other sweeteners will change the taste; even little amounts of extra sweeteners will significantly diminish the faint bitter metallic aftertaste of saccharin. Saccharin coupled with aspartame, cyclamate, thaumatin, or xylit is commonly used in the food business for this purpose. Saccharin is best used as a sugar substitute for diabetics or overweight people because it is usually eliminated intact through urine. The maximum daily intake is equal to 5 mg per kilogramme of body weight. Saccharin is found in cosmetics, toothpaste and mouthwash, pharmaceuticals, cigarettes, as a flavouring agent in pig feed, and even as a brightener in anticorrosive nickel coatings, despite the fact that it is most well-known as a food additive E954.
FAQs
Why do we need artificial sweeteners in the first place?
Artificial sweetening agents are required since they have a sweet taste but no nutritional benefit. They pass through the gastrointestinal tract without being digested. It allows diabetics to eat sweet foods without putting additional sugar into their bodies.
What is the reason for the prohibition of saccharin?
Saccharin was banned in 1981 due to concerns about carcinogenesis. Consumption of 5 g saccharin every day for 5 months had no adverse effects on humans in an experiment.
Was saccharin's sweetness discovered by chance?
Saccharin, called after the Latin word for sugar, was discovered by accident in 1897 by a researcher from Johns Hopkins University who was exploring new uses for coal tar derivatives. He hadn't washed his hands before lunch and had a sweet flavour on his fingertips.







