Table of Contents
Introduction
Oxides: One or more oxygen atoms are coupled with another element to form oxides, which are chemical compounds (e.g., Li2O). Oxides are oxygen-containing binary compounds with other elements, such as CO2, SO2, CaO, CO, ZnO, BaO2, H2O, and so on. Because oxygen is combined with only one element, these are called oxides. Oxides are characterized as acidic, basic, amphoteric, or neutral based on their acid-base characteristics: Acidic oxide is an oxide that forms an acid when it reacts with water. A basic oxide is an oxide that produces a base in water. A substance that can react chemically as either an acid or a base is known as an amphoteric solution.
A brief outline
Formation of oxides:
Oxygen forms stable chemical connections with practically all elements due to its electronegativity, resulting in oxides. Noble metals (such as gold or platinum) are desired because they resist direct chemical reactions with oxygen, necessitating the production of gold (III) oxide via indirect methods.
Hydrolysis and oxidation by oxygen are two distinct mechanisms for element corrosion. Water and oxygen are even more caustic when combined. Almost all elements burn in an oxygen-rich environment or in an oxygen-rich atmosphere. Some elements, such as sodium, react quickly in the presence of water and oxygen (or just air) to form hydroxides. Alkali and alkaline earth metals are not found in their metallic, or native, state in nature, in part because of this.
Important concepts
In the presence of air, most metals’ surfaces are made up of oxides and hydroxides. Aluminum foil, for example, is covered with a thin layer of aluminum oxide that passivates the metal and slows further corrosion. Electrolytic anodizing can be used to increase the thickness of the aluminum oxide layer. Although solid magnesium and aluminum react slowly with oxygen at STP, they burn in the air, resulting in extremely high temperatures.
Properties of oxides
- On the left of the periodic table, electropositive components can be found. The electropositive characteristic of the elements gradually decreases with time, giving way to elements with an increasing electronegative character.
- The metalloids, which are put between electropositive metals and electronegative non-metals, show how the property changes.
- Elements become more electropositive as they progress down the column, forming stronger basic oxides. In any group, the basic character of the oxides increases down the column. Oxide solubility rises as you go down the column.
- Most elements have many oxidation states, and oxides stabilize the combined element’s maximum oxidation state.
- As a result, the oxide’s stability increases as the oxidation state of the element increases. The oxide is more acidic as the oxidation state of the element increases.
Types of oxides
- Acidic oxide: When non-metals respond with oxygen, they produce acidic oxide buildings connected together by covalent bonds. Corrosive anhydrides are one more name for these synthetics. Aside from substances like B2O3 and SiO2, which have high softening focuses and structure enormous particles, corrosive anhydrides typically have moderate liquefying and edges of boiling over.
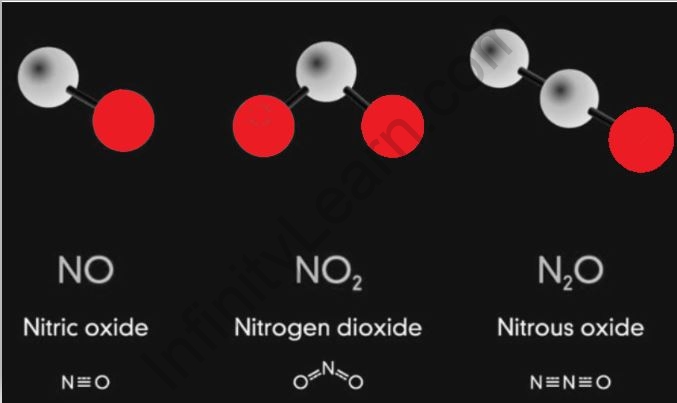
Examples: NO, CO2
SO3 + H2O → H2SO4
- Basic oxide: Fundamental oxide is framed when metals respond with oxygen to shape essential oxygen compounds. Most of these synthetics are ionic in nature. Whenever bunch 1, 2, and lanthanides respond with dioxygen, they produce essential oxygen compounds. A ton of energy is delivered during the amalgamation of these atoms. Aside from a couple of special cases, these synthetic compounds promptly respond with water.
Examples: M2O3, MO2, ThO2
Na2O + H2O → 2NaOH
- Amphoteric oxide: Amphoteric oxides are oxygen-based particles that have both acidic and essential properties. Whenever these oxides consolidate with corrosive, a balance response happens, bringing about the arrangement of water and salt. This exhibits the mixtures’ central property. Like the antacid, it joins with it to create salt and water, giving it an acidic property. Aluminum oxide, for instance.
Acidic characteristics:
Al2O3 + 6HCl → 2Al3+ + 6Cl– + 3H2O
Basic characteristics:
Al2O3 +2OH– + 3H2O → 2[Al (OH)4]–
- Neutral Oxides: When certain substances respond with oxygen, they create oxides that are neither acidic nor essential. The expression “unbiased oxygen compounds” alludes to such substances.
Example: NO, CO.
Uses of Oxides
- It’s used in meatpacking methods that use a modified environment to keep the meat fresh.
- It is employed as a lasing media in elevated infrared lasers.
- Because silicon does not form a double bond with oxygen, it only exists in the form of dioxide. The mineral silica, commonly known as silicon dioxide, is abundant in the earth’s crust. Silica is used in the following cases:
- It is used in the manufacture of glass
- In the food sector, silicon dioxide is used as an ingredient. Silicon dioxide promotes bone density, which helps to prevent disorders like arthritis and osteoarthritis.
- Silica is used in the manufacture of cement, which is a critical component in the building industry.
Uses of Oxides of Germanium
- Amphoteric oxides, like germanium oxide, mix in both acids and bases. It is the primary source of commercially available germanium. The following are some of the applications for germanium dioxide:
- In an optical instrument, germanium dioxide is used.
- It is exploited as a diatom growth blocker, which aids in the production of algae.
Significance of oxides in NEET exam
The audit material for Infinity Learn is conveyed totally by likely the best teacher in the country. Their knowledge and showing style make the survey materials unimaginably profitable in further developing your NEET test execution on this particular point. The justification behind the survey materials is to make huge material in an essential language using crucial approaches that are both productive and careful. Consequently, exactness, speed, and steadiness are totally joined. In like manner, the NCERT Solutions made by staff are the principal piece of any board test, JEE, or NEET test. These courses of action are revived reliably and adhere to the most recent CBSE guidelines and instructive program.
Because of its electronegativity, oxygen can form a stable chemical bond. The matching oxides are formed when oxygen makes a chemical bond with the majority of the elements. Only a few metals cannot be iodized, and these metals are referred to as noble metals. As a result, these metals are also quite expensive. Noble metals include metals such as gold and platinum. They don’t allow for direct chemical reactions with oxygen; thus, they could only be oxides in a roundabout way.
FAQ’s
A binary molecule of oxygen and then another element is known as an oxide. The reactivity of oxygen is very high. When they react with metals and non-metals, they produce oxides. Sulfides are formed from sulfoxides, and amines are formed from amine oxides, wherein the oxygen atom is covalently bonded to the nitrogen or sulfur atom.
In the laboratory, oxides are used to create salts and in the manufacture of slag. Certain oxides are used as a drying agent.
Some oxides are neutral, which means they are neither acidic nor basic in nature. Such oxides are referred to as neutral oxides. Examples are nitrous oxide, water, and nitric oxide. What happens when an oxide is formed?
What is the function of ozone?
What are neutral oxides, but what do they look like?









