Table of Contents
Definition
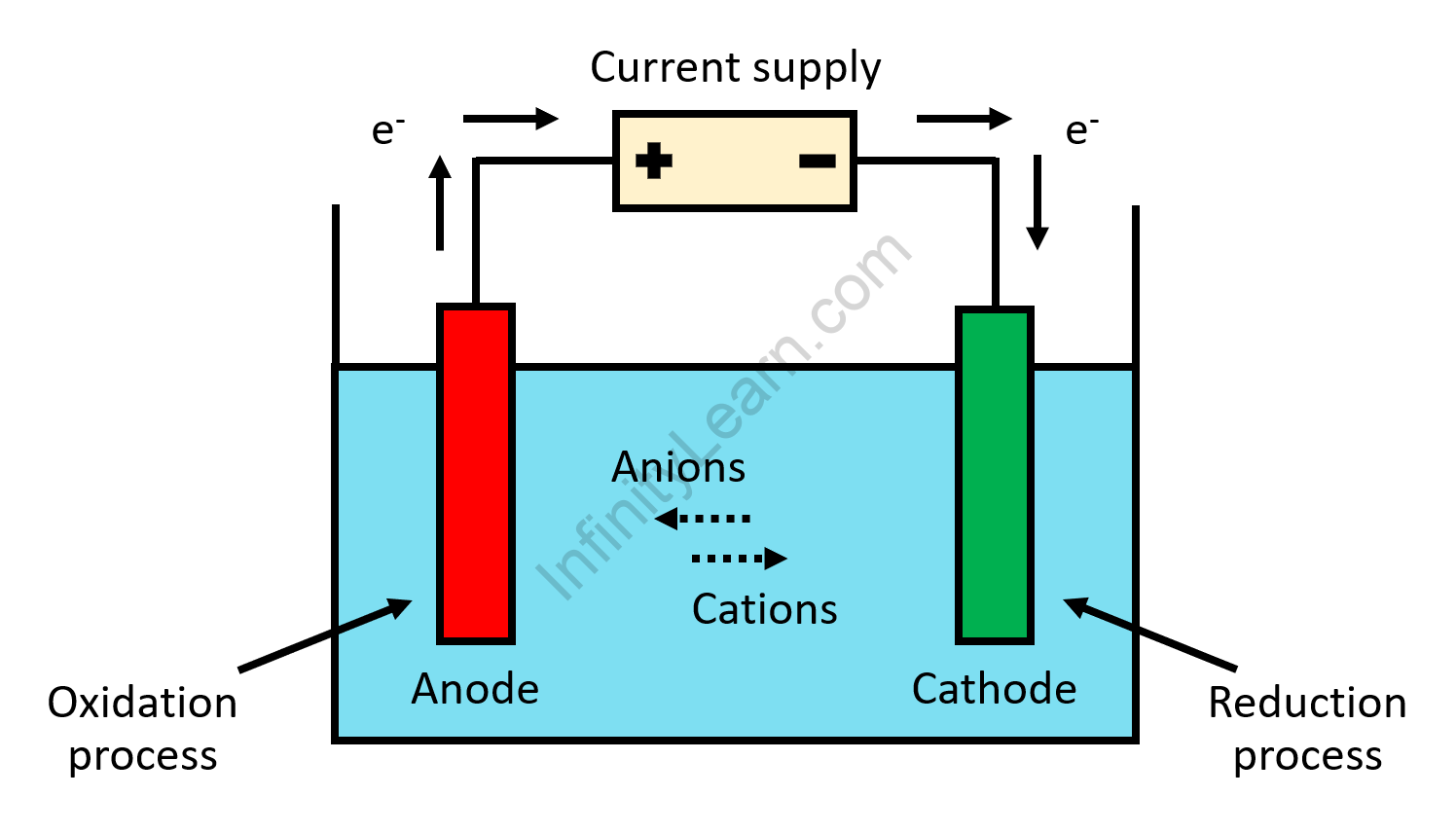
Oxidation is characterized by a loss of electrons, the gain of oxygen, or the elimination of hydrogen. The concept reduction denotes an electron gain, a hydrogen gain, or the elimination of oxygen. The term LEOGER (Loss of Electron Oxidation Gain of Electron Reduction) is used to understand oxidation and reduction easily.
Oxidation and Reduction are defined by two concepts:
- Classical Concept of Oxidation and Reduction
- Electronic Concept of Oxidation and Reduction
What is Oxidation?
- According to the electronic concept it is defined as the process by which an atom or ion loses one or more electrons in an electronic sense.
- Classical or older concepts define oxidation as the removal of hydrogen or an electropositive element or the addition of oxygen or any electronegative element
What is Reduction?
- According to the electronic concept, the process by which an atom or ion obtains one or more electrons is characterized as a reduction.
- Classical or older concepts define reduction as the removal of oxygen or any electronegative element or the addition of hydrogen or an electropositive element.
Difference between Oxidation & Reduction
When a reactant loses electrons during a process, this is referred to as oxidation. When a reactant obtains electrons during a reaction, this is referred to as reduction. This is a common occurrence when metals react with acid. When a reactant loses electrons during a process, this is referred to as oxidation. When a reactant obtains electrons during a reaction, this is referred to as reduction. This is a common occurrence when metals react with acid.
Classical Idea of Oxidation and Reduction reactions:
Oxidation reactions include the following:
- Addition of oxygen
2Mg+O2→2MgO
- Removal of hydrogen
HS+Cl2→HCl+S
- Removal of electropositive elements
2KI+H2O2→2KOH+I2
- Addition of electronegative element
Fe+S→FeS
Reduction reactions include the following:
- Addition of hydrogen
N2+3H2→2NH3
- Removal of oxygen
ZNO+C→Zn+CO
- Removal of electronegative element
2FeCL3+H2→2FeCl2+2HCl
- Addition of electropositive element:
SnCl2+2HgCl2 →SnCl4+Hg2Cl2
Electron Transfer Concept of Oxidation and Reduction reactions
This is the most often used and universally applicable concept of oxidation and reduction.
Oxidation reactions include the following:
Loss of electron:
Mg→ Mg2+2e–
Reduction reactions include the following:
Gain of electrons:
O+2e–→O2-
Interlink between oxidation and reduction:
The processes of oxidation and reduction are inextricably linked. Because electrons are never created or destroyed during a chemical reaction, oxidation and reduction must always take place in pairs; one cannot exist without the other.
A reduction-oxidation reaction, also known as a redox reaction, is a type of chemical reaction in which reduction and oxidation occur simultaneously. The reduced species gains electrons, whereas the oxidized species loses them.
For instance; in the following example Magnesium is oxidized by losing two electrons to oxygen, which is then reduced by getting two electrons from magnesium.
2Mg+O2→2Mg2+O2-
Because oxidation and reduction cannot occur separately, they are referred to together as ‘Redox Reactions.’ The reactant that oxidizes the other reactants is referred to as the Oxidizing agent, whereas the reactant that reduces is referred to as the Reducing agent.
In other words, a material that is oxidized functions as a reducing agent, whereas a substance that is reduced works as an oxidizing agent.
Conclusion:
Oxidation is a chemical reaction in which one or more electrons are lost or the element’s valency rises. Reduction is a process in which one or more electrons are acquired or the element’s valency drops. The oxidizing agent is a substance that can receive one or more electrons, resulting in a drop in valency. A reducing agent is a substance that may lose one or more electrons, causing its valency to rise. A redox reaction consists of two half-reactions, one of which involves the loss of electrons or electrons (oxidation) and the other of which involves the gain of electrons or electrons (reduction).
Oxidation and Reduction are defined by two concepts. The term LEOGER is used to understand oxidation and reduction easily. Oxidation occurs when a reactant loses electrons during a process. Reduction occurs when a reactant acquires electrons during a reaction. Oxidation occurs when a reactant loses electrons during a process. Reduction occurs when a reactant acquires electrons during a reaction.
Also read: Important Topic Of Chemistry: Degree of Ionization
FAQs
What is the primary distinction between oxidation and reduction?
Reduction is the process of gaining electrons, whereas oxidation is the process of losing electrons.
What is the oxidation state?
The degree of oxidation (loss of electrons) of an atom in a chemical compound is characterized by the oxidation state, often known as the quantity of oxidation.
What is the significance of oxidation and reduction?
The importance of oxidation-reduction (redox) processes cannot be overstated because they are the primary natural, biological, and manmade energy sources on this planet. By removing hydrogen and replacing it with oxygen, oxidation of molecules often releases huge quantities of energy.