Table of Contents
Introduction
Whenever incoming light with an energy larger than the metal’s threshold value strikes the surface, the metal’s tightly bound electrons are released. A picture is a tiny speck of light. If a photon collides with an electron, the sum of its energy is transferred to the electron, causing the electron to eject off the surface. The photon’s remaining energy is converted into a free negative charge known as a photoelectron. The photoelectric effect is a mechanism in which light causes electrons to be expelled from a metal’s surface. Photoelectrons are the electrons that are expelled. The emission of photoelectrons and the kinetic energy of the expelled photoelectrons are affected by the frequency of light incident on the metal’s surface. Photoemission is the word used to describe the phenomenon of photoelectrons being emitted from a metal’s surface as a result of light action. Whenever electrons on the surface of a metal absorb energy from incident light, they use it to overcome the attraction forces that bind them to the nuclei of the metal.
The information about the photoelectric effect and Einstein’s equation of the photoelectric effect from various physics-related articles are available here. Photoelectric effect and its general concepts are important topics in physics. Students who want to flourish in physics need to be well known about the photoelectric effect and Einstein’s equation of the photoelectric effect to get deep knowledge about it to do well on their exams. The definition, brief explanation, and derivation are provided here to assist students in effectively understanding the respective topic. Continue to visit our website for additional physics help.
Overview
Albert Einstein, a German physicist, is regarded as one of history’s greatest scientists. To mention a few fields, he has made contributions to general relativity, black holes, and the photoelectric effect. The Nobel Prize in Physics was presented to Einstein in 1921 for his discovery of the photoelectric effect. Einstein’s concept of light was both new and beautiful. He demonstrated an effective irradiation approach. In general, photons are said to be a small collection of particles that make up light. These particles have a greater energy level, which is referred to as the quantum of radiation. As a result, light is made up of energy packets or quantum.
Photons are particles that carry momentum and energy from the light source from whence they are emitted. Einstein won the Nobel Prize for his discovery of the photoelectric effect, which was one of his greatest achievements. Light was the first to be described as both a wave and a particle, according to Einstein. This is referred to as light’s wave-particle duality. Quantum mechanics’ core principle of wave-particle duality is the driving force behind the development of solar cells and electron microscopes. When a metal surface is bombarded with enough energy light, it causes the metal’s electrons to eject out, according to the Photoelectric effect.
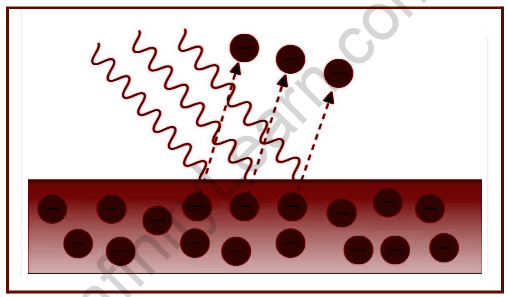
Einstein’s Theory of Photoelectric Effect
Due to the oscillating electric field of the incident light, the electrons within the atoms of the metal surface obtain energy and begin vibrating at a high frequency. When the incident radiation’s energy exceeds the metal’s work function, the electrons receive enough energy to expel from the surface. The colour and intensity of incident radiation, as well as the temporal length of incident radiation, influence the speed and number of released electrons.
- Whenever incident radiation is more intense, electrons receive more energy and vibrate more, resulting in a bigger number of electrons being emitted with a faster average speed.
- Higher-frequency incident radiation causes electrons to oscillate quicker, increasing electron emission. In most cases, dim light does not offer enough energy for electron emission.
- The photoelectric current’s strength is determined by the intensity of incident radiation and should be greater than the threshold frequency.
- The photo-current stop was the reverse stopping possibility. It is unaffected by incident radiation intensity.
- If the frequency of the incident radiation is less than the threshold frequency, photoelectric current does not exist. When exposed to light or the sun, a metallic strip will not be able to produce the Photoelectric effect unless the frequency exceeds the threshold value.
- The photoelectric effect occurs in a split second. The metal’s electrons are ejected as soon as light strikes the surface.
Planck’s Theory and the Photoelectric Effect
In 1905, Einstein modified Planck’s theory to describe the photoelectric effect, which occurs when metal is exposed to light or high photons and releases electrons. The frequency of radiation v determines the kinetic energy of the emitted electrons, not their intensity; for a given metal, there is a frequency 0 below which no electrons are released. Furthermore, emissions happen immediately after the light strikes; there is no apparent delay. According to Einstein, these effects may be described by two assumptions: light is made up of corpuscles or photons, the power of which is specified by Planck’s equation, and a single metal atom can absorb a single photon or anything. Because low-frequency beams could not create the energy needed to produce photoelectrons, as light energy from continuous waves, Albert Einstein argued that light beats were huge packets of varying energies known as photons, rather than a wave moving through the atmosphere.
The frequency of light is equal to the photon energy of light beams of varying intensities. When an electron absorbs photon energy and obtains more energy than its binding force, it is more likely to be freed from the imaging process. When the photon energy is too low, an electron cannot emerge from an object. Because raising the intensity of low-intensity light will simply produce more low-energy photons, no single photon with appropriate electron production will be produced. Furthermore, the individual photon energy determines the amount of electron energy released, not the strength of the incoming light of a specific frequency.
Photoemission can happen in any object, but metals and other conductors are the most common examples. This is because the process creates a cost imbalance, which causes the barrier to rise until the discharge is entirely exhausted if it is not measured by the current flow. Many functional investigations and devices based on the effect of electric photography require clean metal surfaces in the extracted tubes because non-conductive oxide ports on metal surfaces boost the energy barrier in picture extraction. By preventing gases from interfering with electron passage between electrodes, the vacuum also aids electron microscopy.
This photoelectric effect cannot be described using the wave model of light. But, this behaviour can be explained by the particle nature of light, which can be regarded as a stream of electromagnetic energy particles. The visible spectrum is made up of photons, which are light ‘particles.’
The energy held by a photon is related to the frequency of light via Planck’s equation.
It is, E=hv=hc/λ
Here,
- E is treated as the energy of the photon
- h is said to be Planck’s constant
- v can be the frequency of the light
- c is considered as the speed of light (in a vacuum)
- is considered as the wavelength of the light
As a result, it’s easy to see how different light frequencies convey photons with varied energy. Blue light, for example, has a higher frequency than red light (the wavelength of blue light is much shorter than the wavelength of red light). Finally, the energy of a photon of blue light will be higher than that of a photon of red light.
Einstein’s Equation of the Photoelectric Effect
The Einstein-Planck relation states that Einstein explained the photoelectric effect using Planck’s quantum theory, which states that light travels in discrete photons. The energy photon has the formula hv, where h is constant and v is the light frequency.
E=hv…(1)
The Photoelectric effect studies show that if the incident radiation frequency is less than the threshold frequency, no electron emission occurs. Energy is closely connected to frequency, as shown by the equation, which also explains the instantaneous nature of electron emissions.
Because there is no electric field outside the metallic surface, the photoelectron will be transformed to simply kinetic energy when it exits. The electron uses some of the quantum energy provided by photons to overcome the surface’s chemical pull.
As a result, a photoelectron’s kinetic energy is equal to (energy imparted by photon) – (energy used to come out of the surface).
For a surface, this energy is constant and is denoted by . It is called a surface’s work function, and it is constant for any given material.
K.E.=hv-Φ…(2) ,
which is Einstein’s photoelectric equation.
The photoelectrons are in the same boat. To be ejected from the surface, electrons must have minimum threshold energy. Electrons gain enough energy to eject from the surface when they are given a threshold frequency (v0). After emerging out of the surface, if the electron gains energy equal to the threshold frequency, its kinetic energy is zero. Finally, we have
hv0 -Φ=0 or hv0 =Φ…(3)
Applying this in equation (2), we get
K.E. = hv – hv0 or K.E.=h(V-v0)
And, v0 is treated the Stopping Potential,
Now,
K.E. (max)= eV0;
When putting this in equation (3), we get
eV0 =h(v-v0)…(4)
The value of ‘h’ for the Photoelectric effect is found using this equation. This equation yields numbers that are consistent with original values, confirming Einstein’s theory of the Photoelectric Effect.
Frequently Asked Questions (FAQs)
How can the photoelectric effect be understood?
The easiest way to comprehend the photoelectric effect is to look at the atom's line spectrum.
What are the known applications of the Photoelectric Effect?
The photoelectric effect is used directly in photocells and solar cells. The quantum revolution was sparked by the photoelectric effect. Scientists briefly studied the nature of light and the structure of atoms, and as a result, the physical foundation of the entire world.
What is the importance of the photoelectric effect?
Our understanding of the quantum nature of light and electrons has grown as a result of research into the photoelectric effect. It has further affected the development of the wave-particle duality notion. The photoelectric effect is also commonly used to look at the energy levels of electrons in substances.
What causes the Auger effect?
When an electron beam collides with a surface, it forms electron holes in the bottom shell, which are then filled by electrons from the upper shell. This jump's surplus energy is transferred to another electron and then released.
What is the work function of the Photoelectric Effect?
Take a ping pong ball and place it inside a bucket. If we strike the ball with other small balls from outside, the vibration of the ball will rise, and it will come out. The smaller ball should have enough energy to pop the ball out, and this energy is referred to as the bucket and ball's work function.









