Table of Contents
Introduction
Boiling point elevation definition: The temperature during which the pressure is exerted by the surroundings on a liquid is matched by the pressure exerted by the liquid’s vapor; under this situation, adding heat causes the liquid to change into its vapor without raising the temperature. The vapour pressure rises as the temperature rises; at the boiling point, vapor bubbles form inside the liquid and come to the surface. The normal boiling point of a liquid is the temperature at which the vapor pressure equals the standard sea-level air pressure; the boiling point of a liquid varies depending on the applied pressure. Water boils at 100° C at sea level. The boiling point temperature falls at greater elevations.
A brief outline
The term “saturation temperature” refers to the temperature at which water boils. The saturation temperature is the temperature at which a liquid boils into the vapor phase at certain saturation pressure. The thermal energy in the liquid is considered to be saturated. A phase change occurs when thermal energy is added. A vapor at saturation temperature will begin to condense into its liquid phase as thermal energy (heat) is withdrawn if the pressure in the system remains constant (isobaric). Similarly, as more thermal energy is provided to a liquid at saturation temperature and pressure, it will boil into the vapor phase. The boiling point is the temperature at which the liquid’s vapor pressure matches the surrounding atmospheric pressure. As a result, the boiling point is influenced by pressure.
The boiling point of a fluid is affected by impurities, which raise it. When an impurity is introduced to a liquid, the vapour pressure of the liquid will likely fall, raising the Boiling Point. For example, if you boil water on the stove and add even a pinch of salt, the water’s boiling point will rise. Impurities, on the other hand, only serve to lower the melting point of a liquid.
Important concepts
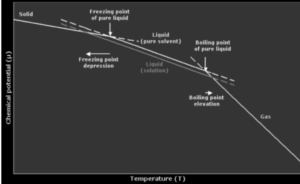
Boiling water Elevation:
When another component is added to a liquid (a solvent), the boiling point of the liquid (a solvent) increases, implying that a solution has a higher boiling point than a pure solvent. When a non-volatile solute, like salt, is supplied to a solvent, such as water, these results. An ebullioscopy can be used to precisely measure the boiling point.
The boiling point elevation is a colligative attribute, meaning it is affected by the presence and amount of dissolved particles, but not by their identity. It’s a result of the solvent being diluted in the presence of a solute.
Elevation of boiling water formula
The boiling point of a non-volatile solute solution can be expressed in terms: The boiling point of a solution is equal to the boiling point of the pure solvent plus the elevation of the boiling point (∆Tb). The concentration of the solute in the solution is proportional to the increase in boiling point (∆Tb). The following equation can be used to compute it.
ΔTb = i×Kb ×m
Where,
- i = Van’t Hoff factor
- Kb = ebullioscopic constant
- m = molality of the solute
It’s worth noting that when the solute concentration is really high, this formula becomes less exact. In addition, this formula does not apply to volatile solvents. The ebullioscopic constant (Kb) is commonly represented as degrees Celsius per molal or degrees Celsius per kilogram of mol-1
Use of boiling point elevation
The degree of breakdown or the molar mass of the solute can be calculated using the boiling point election and formulas for boiling point elevation. Ebullioscopy, a Greek phrase for boiling watching, is the name given to this sort of measurement. The cryoscopic constant, on the other hand, states that the freezing point depression is greater than the ebullioscopic constant. However, the freezing point is simple to calculate and is extensively employed in cryoscopy.
Elevation of boiling water derivation
The vapor pressure of a substance increases with increasing temperature. At a temperature where the vapor pressure equals the air pressure, a liquid boils. In the presence of a non-volatile solute, the solvent’s vapor pressure drops.
A solution’s boiling point is always higher than the boiling point of the pure solvent in which the solution is made. The number of solute molecules, rather than their composition, determines how high the boiling point rises.
∆Tb = Tb – Tb0 is the increase in boiling point. This is referred to as boiling point elevation.
Tb0 = Pure solvent boiling point
Tb0 = Boiling point.
The increase in boiling point Tb in dilute solutions is proportional to the molal concentration of the solute in the solution.
ΔTb ∝ m
ΔTb = kbm
Significance of boiling point in NEET exam
The master or educator of infinity learn places forward their maximum effort consistently to make their notes, and they guarantee that all perspectives are tended to completely so understudies get the most noteworthy potential imprints on their NEET test. Model inquiry responses to are portrayed exhaustively with impeccable explanations for every section to guarantee that understudies get the thought. Every number-related issue is settled each in turn, with no progression bounces, which is simple for youngsters to acknowledge. Understudies taking NEET tests will benefit extraordinarily from the components recorded previously.
FAQ’s
Cooking is one instance where the boiling point elevation can be used. Many recipes call for salt to be placed in water before it boils in order to season it, although this isn't necessary. As a result, if you salt the water, its boiling point will rise, and it will take longer for the water to boil than usual.
If you boost the boiling point, you'll be more protected from boilovers. You'll notice that there are a variety of antifreeze brands that will tell you how much boil-over and freeze-up protection they provide. Through the freezing point depression, the antifreeze will help to keep the water in your vehicle's radiator from freezing.
Physical features of a solution such as boiling point elevation and freezing point depression are known as collective properties. However, as we add additional solute to the solvent, the temperature of the solution varies, resulting in a change in the volume of the solution. Because molarity is defined as moles of solute per liter of solution, it cannot be used as a unit of concentration. In real life, how can we use boiling point elevation?
Why do you believe the rise of the boiling point is significant?
Why do you believe that molality is utilized to describe colligative properties?









