Table of Contents
Enzyme Action
INTRODUCTION
The human body is made up of many types of cells, tissues, and other complex organs. In order to function properly, our body releases certain chemicals to speed up biological processes such as respiration, digestion, excretion, and a few other metabolic functions to maintain good health. Thus, enzymes are essential for all living organisms that regulate all biological processes.
Many protein enzymes have the ability to perform various processes. Metabolic processes and other chemical reactions in a cell are made into a set of enzymes needed to sustain life.
The first phase of the metabolic process relies on enzymes, which react with a molecule and are called the substrate. Enzymes convert substrates into different molecules and are called products.
Enzyme control has become an important factor in clinical diagnosis because of its role in maintaining healthy processes. The macromolecular components of all enzymes contain proteins, with the exception of a class of RNA catalysts called ribozymes. The name ribozyme is derived from the ribonucleic acid enzyme. Many ribozyme molecules are ribonucleic acid molecules, which cause reactions in one of their bonds or among other RNA.
Enzymes are found in all tissues and body fluids. Catalysis of all reactions that occur in metabolic pathways is performed by intracellular enzymes. Enzymes in plasma membranes regulate catalysis in cells as a response to cellular signals and enzymes in the circulatory system that regulate blood clotting. Many of the most important health processes are established in the activities of enzymes.
From the above discussion, we can conclude that enzymes are naturally occurring proteins and that they are involved in establishing various biological and chemical processes. Various models are proposed to understand the mechanism of action of enzymes, such as the Lock and Key hypothesis and the Induced Fit model. The basic understanding is that enzymes have an active site in them and substrates bind to them and then convert into products. In this way, the enzyme can be used repeatedly as it can be used in the reaction. Temperature, pH, and substrate concentration are factors that can affect the level of enzyme activity.
STRUCTURE OF ENZYMES
Enzymes are a series of amino acids, which create a three-dimensional structure. The amino acid sequence determines the formation, which also determines the catalytic activity of the enzyme. When heated, the enzyme structure changes, leading to loss of enzyme activity, which is often associated with temperature.
Compared to its substrates, the enzymes are usually large in varying sizes, ranging from 62 amino acid residues to an average of 2,500 residues found in fatty acid synthase. Only a small portion of the structure is involved in catalysis and is located close to the binding areas. The catalytic domain and the binding domain form an active enzyme site. There are a small number of ribozymes that act as RNA-based biological catalysts. It reacts complexly with proteins.
ENZYME ACTION
In order to understand the mechanism of action of the enzyme and the action of the enzyme, two theories were proposed. Kade:
Lock and Key Hypothesis:
This theory was proposed by Emil Fischer in 1894. This concept helps us to understand how enzymes work. According to this theory, the enzymes and molecules of the substrate represent various geometric shapes and these shapes are very clear. Like the key and the key model, this theory states that active enzyme sites act as a key containing certain molecules, such as -O COOH and -SH. These enzyme molecules can only be opened with the help of certain substrate complexes. This hypothesis describes the specification of enzymes and the mechanism of action of enzymes. The substrate mixes with the active site of the enzyme complex and forms an enzyme-substrate complex. When this complex is formed, it undergoes chemical reactions, and finally, the product is formed. Once this product is built, it no longer enters the working area and escapes to the surrounding area. In this way, the active site is also available on new substrates. With this in mind, it can be concluded that a very small amount of the enzyme can work on large substrate molecules. It also explains how enzymes are not used in the reaction and can be used over and over again. In addition, it helps us to understand how to prevent competition.
Fit Hypothesis made:
Koshland proposed this idea in 1960. This view is actually very different from the previous view. It states that the active site of the enzyme is flexible and can change its shape in the form of a substrate, which means that it can form its own active site associated with the substrate. It is easy to understand how the hand causes changes in the glove, that is, in the same way, the active area makes the change in the chemical substrate. The substrate enters the active site of the enzyme. According to this, the structure of the active enzyme varies. There are two types of groups present in the active enzyme area. One is a buttressing group and the other a catalytic group. The buttressing group helps to support the substrate, while the catalytic group helps to explain the mechanism of enzyme catalyzes. When the buttressing group meets the substrate, changes occur in the active environment and these changes help to bring the catalytic group into contact with the substrate bonds needed to be broken. The two models above help us to understand in-depth and explain how the enzyme works.
Mechanism of Enzyme Catalysis
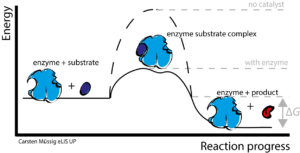
Enzymes are responsible for delivering high levels of chemical conversion. Substrates are converted into products. The substrates bind to the active site of the enzyme, and then there are changes in the enzyme complex, which bring about changes in the enzyme-substrate complex, thus, the product is formed from the substrate. This enzyme-substrate complex is formed in a very short time and is thus known as a transient substance. A temporary structure is formed when a substrate is bound to the active site of the enzyme. Then, the making/breaking of the bonds occurs and the final product is created. Start-up strength is needed to start the reaction. Enzymes work by lowering these high potentials and activating reactions.
The nature of the action of the Enzyme
There is the presence of an active site in each molecule of the enzyme. The substrate mixes with the enzyme and forms an enzyme-substrate complex. After a while, the complex of the enzyme-substrate turns into an enzyme-product complex, and the product is broken down into it. In this way, the enzyme is not used in the reaction. The catalytic cycle helps to explain the mechanism of action of an enzyme by using the following points:
- The substrate is bound to the active site.
- This causes a change in the structure of the enzyme.
- The enzyme-product complex is made by making and breaking bonds.
- The enzyme is released far from the product and is also available through a new set of substrates.
Factors affecting the mechanism of Enzyme Catalysis
There are three factors responsible for affecting the enzyme catalysis process:
Temperature:
Enzyme catalysis works at low temperatures. The best temperature is the temperature at which enzymes show significant catalytic activity. Anything above and below the right temperature reduces the activity of the enzyme. Low temperatures degrade enzymes, while high temperatures change the composition of enzymes.
Hydrogen Ion Concentration:
As there is a high temperature required for the enzyme to work, there is also a concentration of high pH. Any fall or increase in pH reduces the activity of enzymes. Some enzymes show good catalytic activity in the acidic medium, while others show good activity in the alkaline medium. Every enzyme has a high pH, at which point its activity is high.
Substrate Concentration:
Substrates that work on enzymes and then convert them into products. Increased substrate concentration causes an increase in the speed of enzymes.
Mechanism of Enzyme Action in Biochemistry
There are two types of changes observed in chemical compounds, namely, mutations and chemical changes. Physical changes occur only by changing the shape of the compound. There are no restrictions on physical changes. Although, in chemical reactions, new bonds are formed and broken during the conversion process. When ice melts into water, it is called a body transformation as it changes shape. Hydrolysis of starch into glucose is a type of chemical transformation. The level of both physical and chemical processes is the amount of product produced per unit of time. When the direction is specified in the measurement, it is called the velocity. Temperature influences the rate of chemical and physical processes. Different types of enzymes exist and each has a unique catalytic function or a different chemical or dynamic reaction. A metabolic pathway is a process in which a single enzyme stimulates a reaction through different stages. When there are one or two additional reactions to the metabolic pathway, it triggers a variety of final products. Therefore, this helps us to understand how the enzyme works in biochemistry.