Table of Contents
Catalysis is a phenomenon in which the pace of a reaction is influenced by using a material known as a catalyst (the catalyst does not participate in the reaction; its concentration and composition remain unchanged). A catalyst is a material that is used to influence the pace of a process. Enzymes are a type of catalyst that is responsible for enabling and speeding up many important metabolic events in plants and animals. Enzyme catalysis refers to catalysis in which enzymes function as a catalyst.
Enzymes are nitrogen-containing complex molecules. These substances are naturally produced in the bodies of animals and plants. Enzymes are proteins with a large molecular mass that, when dissolved in water, generate a heterogeneous mixture. These proteins have a high efficiency of action and are responsible for a variety of events that occur in the bodies of living creatures.
Enzyme catalysis characteristics
- A single molecule of the enzyme catalyst can change up to a million molecules of the reactant every second. As a result, enzyme catalysts are considered to be extremely efficient.
- These biological catalysts are specific to specific types of reactions, which means that the same catalyst cannot be utilized in more than one reaction.
- A catalyst’s efficacy is greatest at its optimal temperature. At either end of the temperature range, the activity of the biological catalysts decreases.
- The pH of the solution affects biochemical catalysis. A catalyst operates best at an optimal pH, which is between 5-7.
- In the presence of a coenzyme or an activator, such as Na+ or Co2+, the activity of the enzymes normally rises. The presence of a weak link between the enzyme and a metal ion boosts the pace of the reaction.
Many investigations have been done to investigate the processes behind enzyme catalysis. Many ideas have been proposed to explain the interaction between the substrate and the enzyme. Among these research, two well-known ideas are stated below:
- Lock and Key Model: The substrate is depicted in this concept as fitting into the active site in the same way that a key fits into a lock.
- Induced Fit Model: The enzyme is shown in the model as a flexible active site that changes form to accommodate the substrate and promote the reaction.
Mechanism of the enzyme catalyst
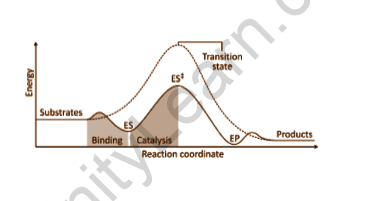
Enzymes are made up of a number of cavities on their exterior surface. These cavities include groups such as -COOH, -SH, and so on. These are referred to as the biological particle’s activity centers. The substrate, which has the opposite charge as the enzyme, fits into the cavities in the same way as a key fits into a lock. Because of the presence of active groups, the generated complex decomposes to give the products.
As a result, this occurs in two steps:
Step 1: Combination of enzyme and reactant
E+R→ER
Step 2: The complicated molecule is disintegrated to provide the product.
ER→E+P
In chemistry, the catalytic action of enzymes has been explained by a variety of processes, depending on the sort of reaction in which they are engaged. They can be of the following varieties:
- Acid-base catalysis: In general, acid catalysis involves partial proton transfer from a donor, which lowers the free energy of the transition state, whereas base catalysis involves partial proton abstraction from a free energy lowering acceptor, which lowers the free energy of the transition state. Acid-base catalysis is useful in biological processes such as peptide and ester hydrolysis, tautomerization, the reactivity of phosphate groups, and addition to carbonyl groups. Asp, Glu, His, Cys, Tyr, and Lys residues are commonly involved in these reactions. Many enzymes use a coordinated acid-base mechanism (i.e., both acid and base catalysis).
- Covalent Catalysis: By creating covalent bonds between enzyme and substrate, covalent catalysis allows for the fast advancement of processes. Covalent catalysis involves three stages: nucleophilic interaction between enzyme and substrate, electrophilic extraction of electrons from the substrate, and elimination reaction.
- Metal Ion Catalysis: Metal Ion Catalysis improves the process in three ways: attaching to and orienting reaction substrates, mediating redox reactions through changes in oxidation state, and electrostatic stabilization or shielding of negative charges. Metal ion-dependent enzymes are classified into two types: metalloenzymes, which contain tightly bound transition metal ions (e.g., Fe2+, Fe3+, Cu2+, Zn2+, Mn2+, and Co3+), and metal-activated enzymes, which contain loosely bound metal ions (e.g., alkali or alkaline metals such as Na+, K+, Mg2+, and Ca2+). Metals can operate as superacids in acid-catalyzed processes, comparable to protons, and are even more effective than protons due to their high pH and charge. Metals shield or lower the effective charge existing on strongly anionic substrates, allowing the nucleophile to contact the substrate during a reaction.
- Electrostatic Catalysis: Electrostatic catalysis occurs in enzymes that appear to organize active site charge distributions to stabilize catalyzed reaction transition states. Substrate binding often prevents water from an enzyme active site, resulting in a low dielectric constant inside the active site. Because of the close proximity of charged groups, the electrostatic interactions in this catalysis are highly strong, and the pKa values can vary by several pH units. In another type of electrostatic catalysis, enzymes employ the charge distribution of the substrates to drive them to their active areas. Other enzymes may have faster response rates than the apparent diffusion-regulated unit, which is also known as substrate channeling.
- Effects of Proximity and Orientation: Substrate binding in this form of enzyme catalysis has additional effects that increase reaction rates. In order for a reaction to occur in this form of catalytic reaction, the reactants must be arranged in the right spatial arrangement and relationship with regard to the enzyme. When comparing similar intermolecular and intramolecular processes, proximity effects are more easily detected.
- Preferential Transition State Binding: In this enzyme catalysis process, enzymes bind to the transition state with more affinity than the substrate or product. This hypothesis explains how products are released into the final solution. Furthermore, it explains the distinction between excellent and bad competitive inhibitors in terms of proximity and orientation effects, which account for the increased rate of responses. According to this idea, enzymes mechanically stretch substrates toward transition states. This is sometimes referred to as the rack mechanism. The rate increase in these sorts of reactions may be represented in terms of enzyme affinity for the transition state relative to the substrate.
Examples of Enzyme Catalysis:
- Manufacture of ethyl alcohol: Ethanol is created from glucose by enzymatic activity. Glucose is transformed into ethyl alcohol and CO2 through the catalysis of the yeast enzyme zymase.
- Urea hydrolysis: Urea is an excretory molecule generated by a live organism’s metabolic processes. It is hydrolyzed to produce ammonia and CO2. Because of the foul odor of NH3, public restrooms frequently stink. The urease enzyme’s enzymatic activity catalyzes this breakdown.
FAQ’s
What is the ideal temperature and pH for an enzyme's action?
Enzymes are destroyed and their function is slowed at high temperatures. Similarly, they are triggered at extremely low temperatures. The ideal temperature for enzyme action is 25 – 55°C, while the optimum pH for enzymatic action is 7.2 – 7.4.
What function do enzyme inhibitors play in enzyme catalysis?
Chemical compounds, bacteria, viruses, and pesticide molecules are the most common enzyme inhibitors. These attacks the enzyme's activation site in two ways. Some compounds function as competitive inhibitors, while others attack the allosteric region of the enzyme, halting its action and lowering its activity.






