Table of Contents
Catalysis of chemical processes is the use of a catalyst to accelerate the pace of a chemical reaction. Catalysts speed up a process without causing any chemical or physical changes. Heterogeneous catalysis offers several advantages. The catalyst in homogeneous catalysis mixes into the reaction mixture, providing for a high degree of contact between the catalyst and reactant molecules. However, unlike heterogeneous catalysis, the homogeneous catalyst is frequently irrecoverable once the reaction has completed.
Catalysis
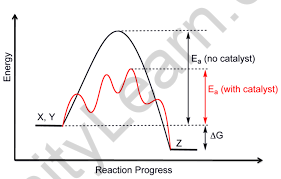
In order to produce products, all reactants must overcome a specific amount of energy known as activation energy. This activation energy is equal to the difference between the energy of the transition state and the energy of the reactant species. Some reactant molecules have enough kinetic energy to break through this energy barrier, whereas others do not.
As a result, in general, not all responses occur at the same rate. As a result, some reagents are added to reduce the activation energy required for the conversion of reactants to products. These compounds are referred to as catalysts, and the process of reducing the activation energy is referred to as catalysis.
Catalysis of chemical processes is the use of a catalyst to accelerate the pace of a chemical reaction. Catalysts speed up a process without causing any chemical or physical changes.
Types of Catalysis:
Catalysis of chemical processes is broadly classified into two types:
- Homogeneous Catalysis: Homogeneous catalysis of chemical processes is a procedure in which the reactants and catalyst are in the same phase. For example, in the presence of sulphuric acid, sugar hydrolysis.
- Heterogeneous Catalysis: Heterogeneous catalysis of chemical processes is a process in which the reactants and catalysts are in distinct stages. For example, in the presence of finely separated iron, hydrogen and nitrogen combine to generate ammonia.
Homogeneous catalysis
The catalyst is in the same phase as the reactants in this case. Typically, everything is present as a gas or in a single liquid phase.
Homogeneous catalysis includes acid catalysis, organometallic catalysis, and enzymatic catalysis. The most common method of homogeneous catalysis is to introduce an aqueous phase catalyst into an aqueous solution of reactants. Acids and bases are frequently highly effective catalysts in these situations because they may speed up processes by influencing bond polarization.
The catalyst in homogeneous catalysis mixes into the reaction mixture, providing for a high degree of contact between the catalyst and reactant molecules. However, unlike heterogeneous catalysis, the homogeneous catalyst is frequently irrecoverable once the reaction has completed.
The following are some frequent instances of homogeneous catalysis reactions:
- A common kind of carbonylation is hydroformylation, which includes the addition of H and “C(O)H” across a double bond. This procedure is nearly entirely carried out with soluble rhodium- and cobalt-containing complexes.
- Homogeneous catalysts are also employed in several oxidations. Acetaldehyde is generated via the Wacker method from ethene and oxygen. Many non-organometallic complexes are also commonly utilized in catalysis, for example, to produce terephthalic acid from xylene. Metal complexes epoxidize and dihydroxylation alkenes, as demonstrated by the Halcon process and the Sharpless dihydroxylation.
Heterogeneous catalysis
- This includes using a catalyst in a separate phase than the reactants. Typical instances utilize a solid catalyst and either liquids or gases as reactants.
- The majority of heterogeneous catalysis instances go through the same stages:
- At active sites, one or more of the reactants are adsorbed on the surface of the catalyst. Adsorption occurs when something adheres to a surface. It is not the same as absorption, which occurs when one substance is taken up by the structure of another. An active site is a portion of the surface that is particularly effective in adsorbing items and assisting them in reacting.
- The surface of the catalyst interacts with the reactant molecules, causing them to become more reactive. This might be a physical interaction with the surface or a weakening of the bonds in the connected molecules. The reaction occurs.
- At this point, one or both of the reactant molecules may be attached to the surface, or one may be attached and struck by the other, which is free to move in the gas or liquid. The molecules of the product are desorbed. Desorption simply refers to the separation of the product molecules. This frees up the active site for a fresh group of molecules to connect to and react with. A good catalyst must adsorb the reactant molecules firmly enough for them to react, but not so strongly that the product molecules adhere to the surface permanently.
- Silver, for example, is ineffective as a catalyst because it does not establish strong enough bonds with reactant molecules. Tungsten, on the other hand, is ineffective as a catalyst because it adsorbs too strongly.
- Metals such as platinum and nickel make excellent catalysts because they adsorb strongly enough to retain and activate the reactants while allowing the products to escape.
The following are some frequent instances of heterogeneous catalysis reactions (reactions in which the physical states of the reactants and the catalysts differ).
- The contact process for the manufacture of sulfuric acid involves the reaction of oxygen and sulfur dioxide catalyzed by vanadium oxides.
- The Haber-Bosch method for industrial ammonia generation utilizes the reaction of hydrogen and nitrogen catalyzed by iron oxides on alumina.
- The Ostwald method, which includes the interaction of ammonia and oxygen catalyzed by an unsupported platinum-rhodium gauze, is used to synthesize nitric acid.
- Steam reforming of methane for hydrogen synthesis, using the chemistry of methane and water catalyzed by nickel or potassium oxide.
The Benefits and Drawbacks of Heterogeneous Catalysis:
Heterogeneous catalysis offers several advantages. For example, heterogeneous catalysts may be easily isolated from a reaction mixture using simple methods such as filtering. This allows for the easy and effective recovery of expensive catalysts, which is a crucial factor for industrial production operations.
One constraint of heterogeneous catalysis is the accessible surface area of the catalyst. When the catalyst’s surface is entirely saturated with reactant molecules, the reaction cannot continue until the products leave the surface and some space opens up for a new reactant molecule to adsorb, or attach.
As a result, the adsorption stage in a heterogeneously catalyzed process is frequently the rate-limiting step. Despite this, the benefits of heterogeneous catalysis frequently outweigh the drawbacks, as the catalyzed process is still significantly quicker than the uncatalyzed reaction.
FAQ’s:
What exactly is auto-catalysis?
Auto-catalysis occurs when one of the products of a process acts as a catalyst for that particular reaction. For example; ester hydrolysis.
How does a catalyst work?
A catalyst is a material that speeds up a chemical reaction without being consumed in the process. A catalyst operates by directing the reaction along an alternative path, one with lower activation energy than the uncatalyzed pathway.









